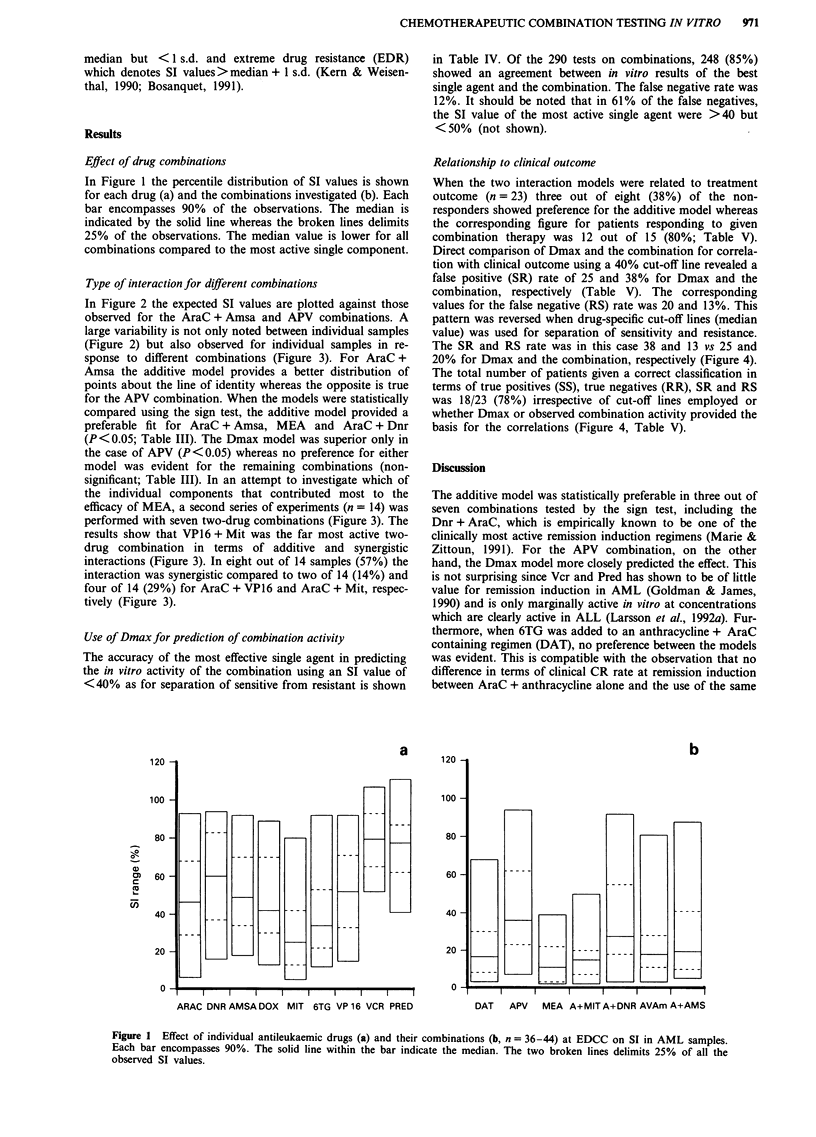
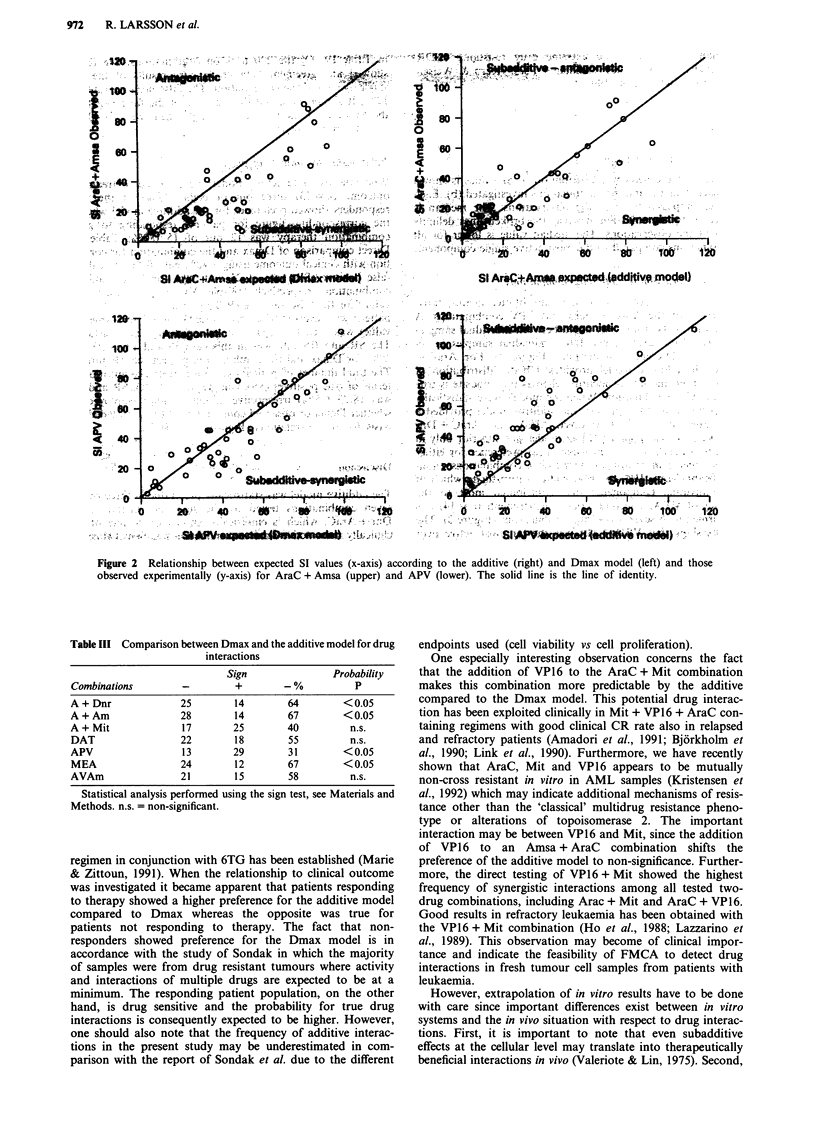
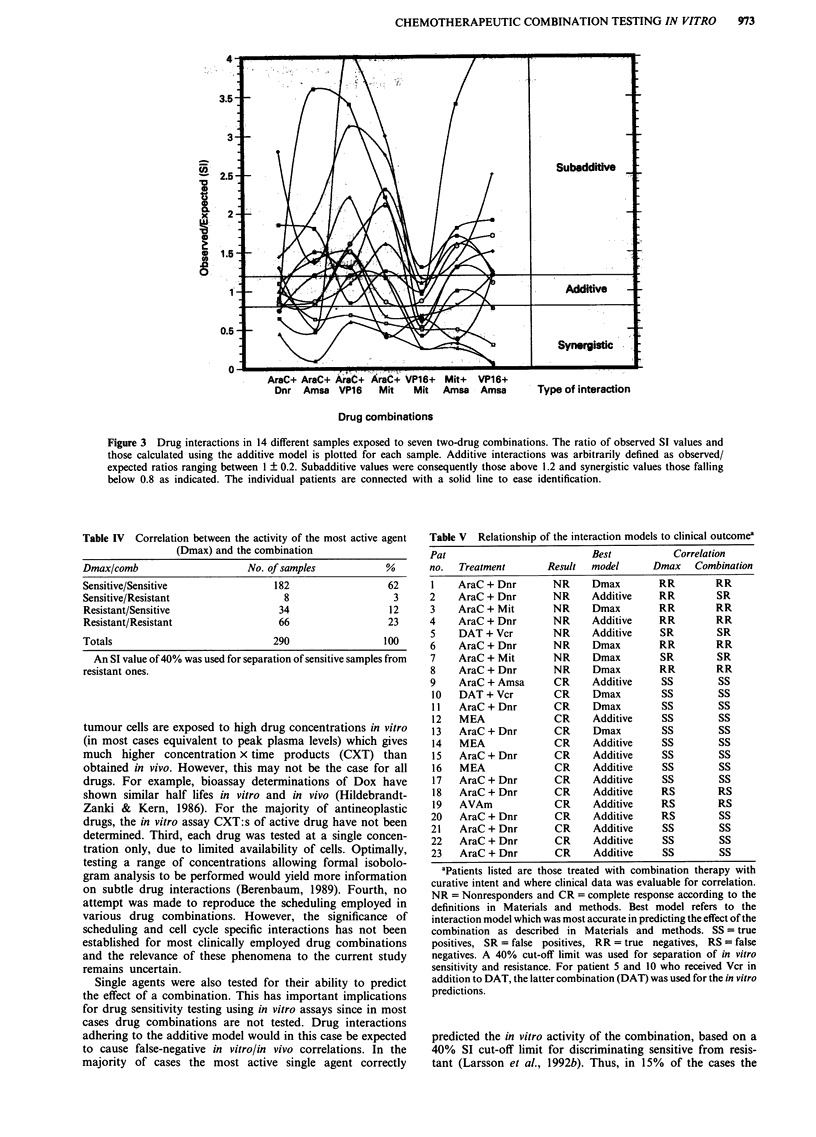
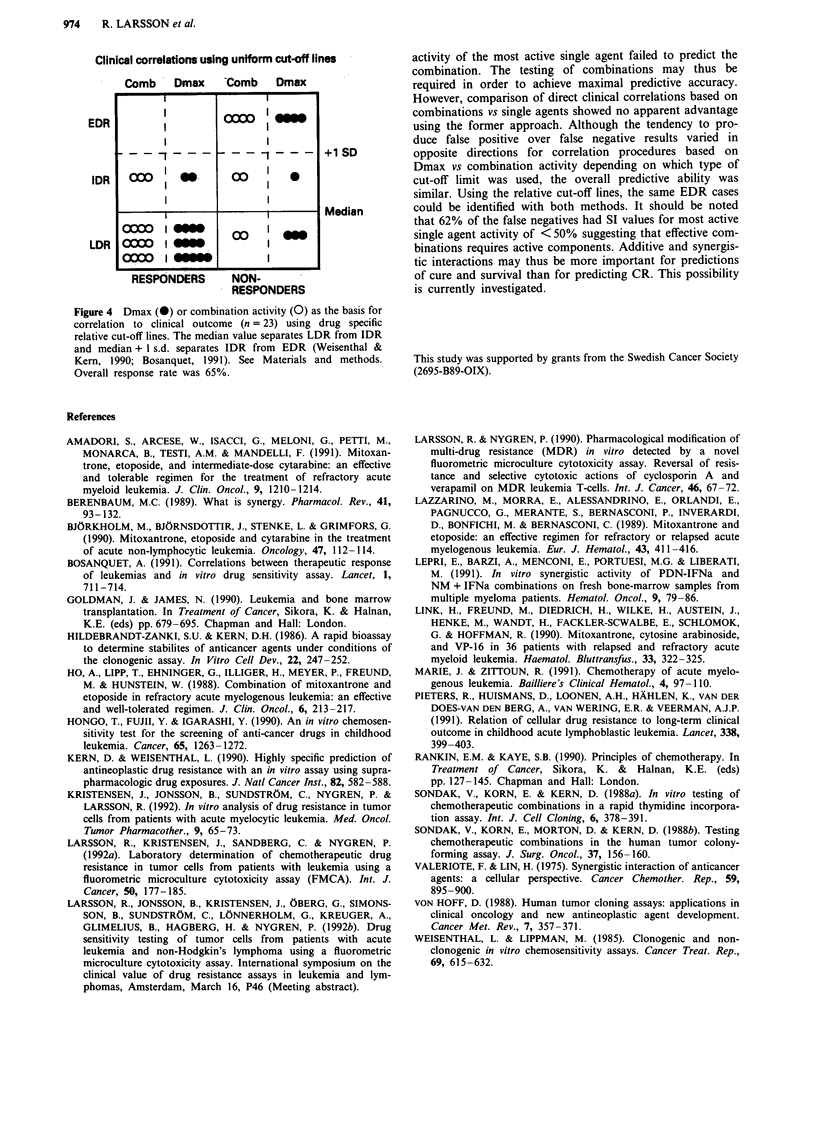
Abstract
The fluorometric microculture cytotoxicity assay (FMCA) was employed for analysing the effect of different chemotherapeutic drug combinations and their single constituents in 44 cases of acute myelocytic leukaemia (AML). A large heterogeneity with respect to cell kill was observed for all combinations tested, the interactions ranging from antagonistic to synergistic in terms of the multiplicative concept for drug interactions. However, an 'additive' model provided a significantly better fit of the data compared to the effect of the most active single agent of the combination (Dmax) for several common antileukaemic drug combinations. When the two interaction models were related to treatment outcome 38% of the non-responders showed preference for the additive model whereas the corresponding figure for responders was 80%. Overall, in 248 of 290 (85%) tests performed with drug combinations, there was an agreement between the effect of the combination and that of the most active single component. Direct comparison of Dmax and the combination for correlation with clinical outcome demonstrated only minor differences in the ability to predict drug resistance. The results show that FMCA appear to report drug interactions in samples from patients with AML in accordance with clinical experience. Furthermore, testing single agents as a substitute for drug combinations may be adequate for detection of clinical drug resistance to combination therapy in AML.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amadori S., Arcese W., Isacchi G., Meloni G., Petti M. C., Monarca B., Testi A. M., Mandelli F. Mitoxantrone, etoposide, and intermediate-dose cytarabine: an effective and tolerable regimen for the treatment of refractory acute myeloid leukemia. J Clin Oncol. 1991 Jul;9(7):1210–1214. doi: 10.1200/JCO.1991.9.7.1210. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C. What is synergy? Pharmacol Rev. 1989 Jun;41(2):93–141. [PubMed] [Google Scholar]
- Björkholm M., Björnsdottir J., Stenke L., Grimfors G. Mitoxantrone, etoposide and cytarabine in the treatment of acute nonlymphocytic leukemia. Oncology. 1990;47(2):112–114. doi: 10.1159/000226800. [DOI] [PubMed] [Google Scholar]
- Bosanquet A. G. Correlations between therapeutic response of leukaemias and in-vitro drug-sensitivity assay. Lancet. 1991 Mar 23;337(8743):711–714. doi: 10.1016/0140-6736(91)90287-y. [DOI] [PubMed] [Google Scholar]
- Hildebrand-Zanki S. U., Kern D. H. A rapid bioassay to determine stabilities of anticancer agents under conditions of the clonogenic assay. In Vitro Cell Dev Biol. 1986 May;22(5):247–252. doi: 10.1007/BF02621226. [DOI] [PubMed] [Google Scholar]
- Ho A. D., Lipp T., Ehninger G., Illiger H. J., Meyer P., Freund M., Hunstein W. Combination of mitoxantrone and etoposide in refractory acute myelogenous leukemia--an active and well-tolerated regimen. J Clin Oncol. 1988 Feb;6(2):213–217. doi: 10.1200/JCO.1988.6.2.213. [DOI] [PubMed] [Google Scholar]
- Hongo T., Fujii Y., Igarashi Y. An in vitro chemosensitivity test for the screening of anti-cancer drugs in childhood leukemia. Cancer. 1990 Mar 15;65(6):1263–1272. doi: 10.1002/1097-0142(19900315)65:6<1263::aid-cncr2820650602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kern D. H., Weisenthal L. M. Highly specific prediction of antineoplastic drug resistance with an in vitro assay using suprapharmacologic drug exposures. J Natl Cancer Inst. 1990 Apr 4;82(7):582–588. doi: 10.1093/jnci/82.7.582. [DOI] [PubMed] [Google Scholar]
- Kristensen J., Jonsson B., Sundström C., Nygren P., Larsson R. In vitro analysis of drug resistance in tumor cells from patients with acute myelocytic leukemia. Med Oncol Tumor Pharmacother. 1992;9(2):65–74. doi: 10.1007/BF02989656. [DOI] [PubMed] [Google Scholar]
- Larsson R., Kristensen J., Sandberg C., Nygren P. Laboratory determination of chemotherapeutic drug resistance in tumor cells from patients with leukemia, using a fluorometric microculture cytotoxicity assay (FMCA). Int J Cancer. 1992 Jan 21;50(2):177–185. doi: 10.1002/ijc.2910500204. [DOI] [PubMed] [Google Scholar]
- Larsson R., Nygren P. Pharmacological modification of multi-drug resistance (MDR) in vitro detected by a novel fluorometric microculture cytotoxicity assay. Reversal of resistance and selective cytotoxic actions of cyclosporin A and verapamil on MDR leukemia T-cells. Int J Cancer. 1990 Jul 15;46(1):67–72. doi: 10.1002/ijc.2910460114. [DOI] [PubMed] [Google Scholar]
- Lazzarino M., Morra E., Alessandrino E. P., Orlandi E., Pagnucco G., Merante S., Bernasconi P., Inverardi D., Bonfichi M., Bernasconi C. Mitoxantrone and etoposide: an effective regimen for refractory or relapsed acute myelogenous leukemia. Eur J Haematol. 1989 Nov;43(5):411–416. doi: 10.1111/j.1600-0609.1989.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Lepri E., Barzi A., Menconi E., Portuesi M. G., Liberati M. In vitro synergistic activity of PDN-IFN alpha and NM + IFN alpha combinations on fresh bone-marrow samples from multiple myeloma patients. Hematol Oncol. 1991 Mar-Apr;9(2):79–86. doi: 10.1002/hon.2900090203. [DOI] [PubMed] [Google Scholar]
- Link H., Freund M., Diedrich H., Wilke H., Austein J., Henke M., Wandt H., Fackler-Schwalbe E., Schlimok G., Hoffmann R. Mitoxantrone, cytosine arabinoside, and VP-16 in 36 patients with relapsed and refractory acute myeloid leukemia. Haematol Blood Transfus. 1990;33:322–325. doi: 10.1007/978-3-642-74643-7_60. [DOI] [PubMed] [Google Scholar]
- Pieters R., Huismans D. R., Loonen A. H., Hählen K., van der Does-van den Berg A., van Wering E. R., Veerman A. J. Relation of cellular drug resistance to long-term clinical outcome in childhood acute lymphoblastic leukaemia. Lancet. 1991 Aug 17;338(8764):399–403. doi: 10.1016/0140-6736(91)91029-t. [DOI] [PubMed] [Google Scholar]
- Sondak V. K., Korn E. L., Kern D. H. In vitro testing of chemotherapeutic combinations in a rapid thymidine incorporation assay. Int J Cell Cloning. 1988 Nov;6(6):378–391. doi: 10.1002/stem.5530060603. [DOI] [PubMed] [Google Scholar]
- Sondak V. K., Korn E. L., Morton D. L., Kern D. H. Testing chemotherapeutic combinations in the Human Tumor Colony--Forming Assay. J Surg Oncol. 1988 Mar;37(3):156–160. doi: 10.1002/jso.2930370304. [DOI] [PubMed] [Google Scholar]
- Valeriote F., Lin H. s. Synergistic interaction of anticancer agents: a cellular perspective. Cancer Chemother Rep. 1975 Sep-Oct;59(5):895–900. [PubMed] [Google Scholar]
- Weisenthal L. M., Lippman M. E. Clonogenic and nonclonogenic in vitro chemosensitivity assays. Cancer Treat Rep. 1985 Jun;69(6):615–632. [PubMed] [Google Scholar]
- von Hoff D. D. Human tumor cloning assays: applications in clinical oncology and new antineoplastic agent development. Cancer Metastasis Rev. 1988 Dec;7(4):357–371. doi: 10.1007/BF00051376. [DOI] [PubMed] [Google Scholar]


