Abstract
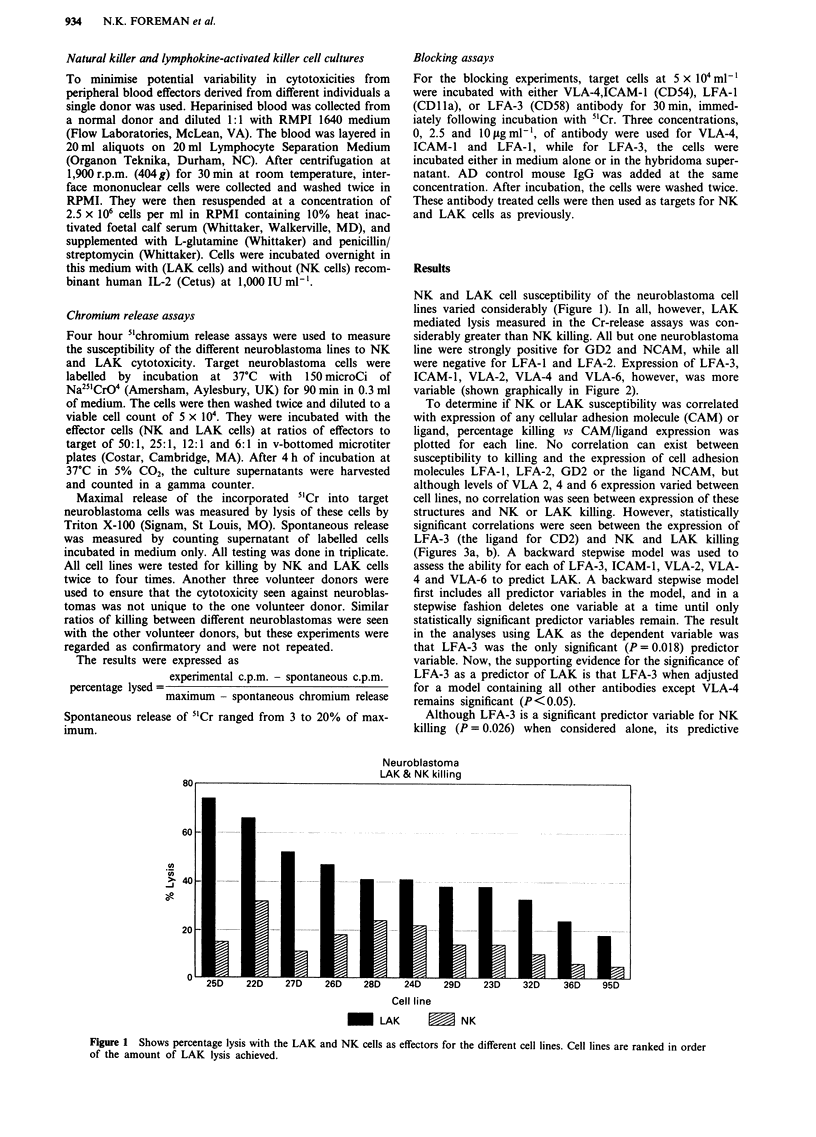
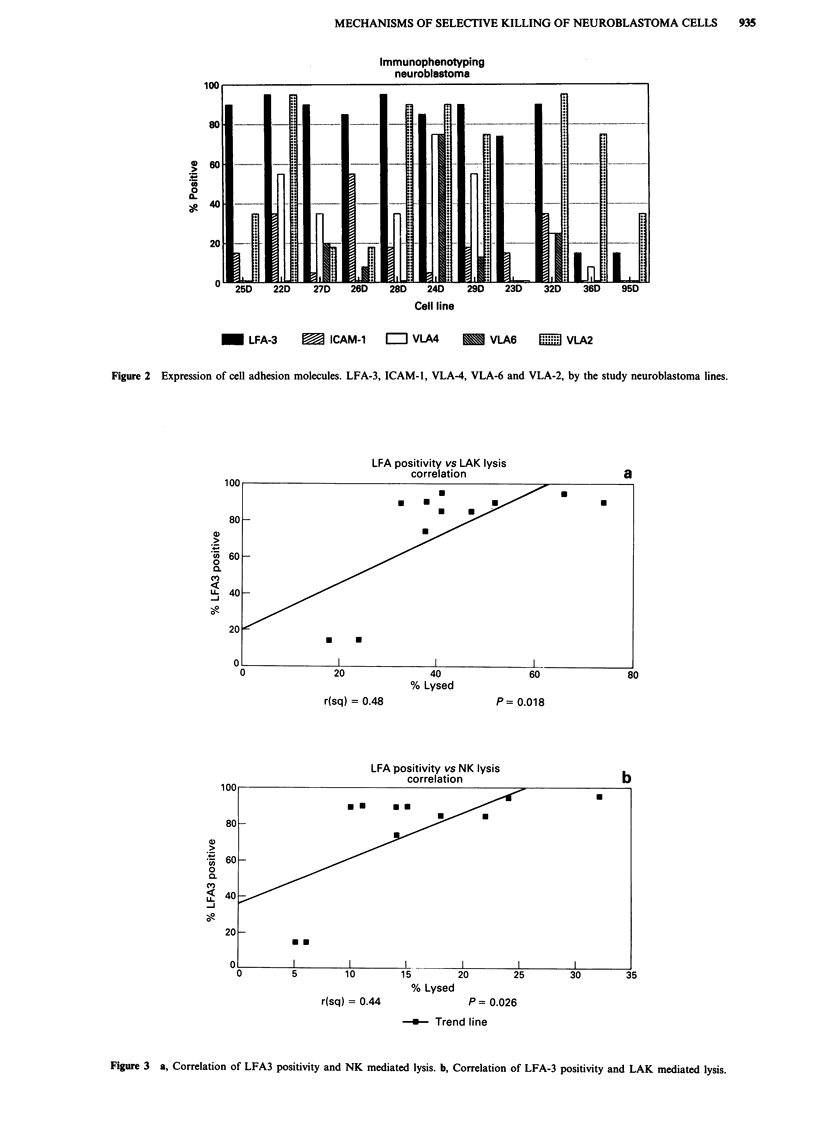
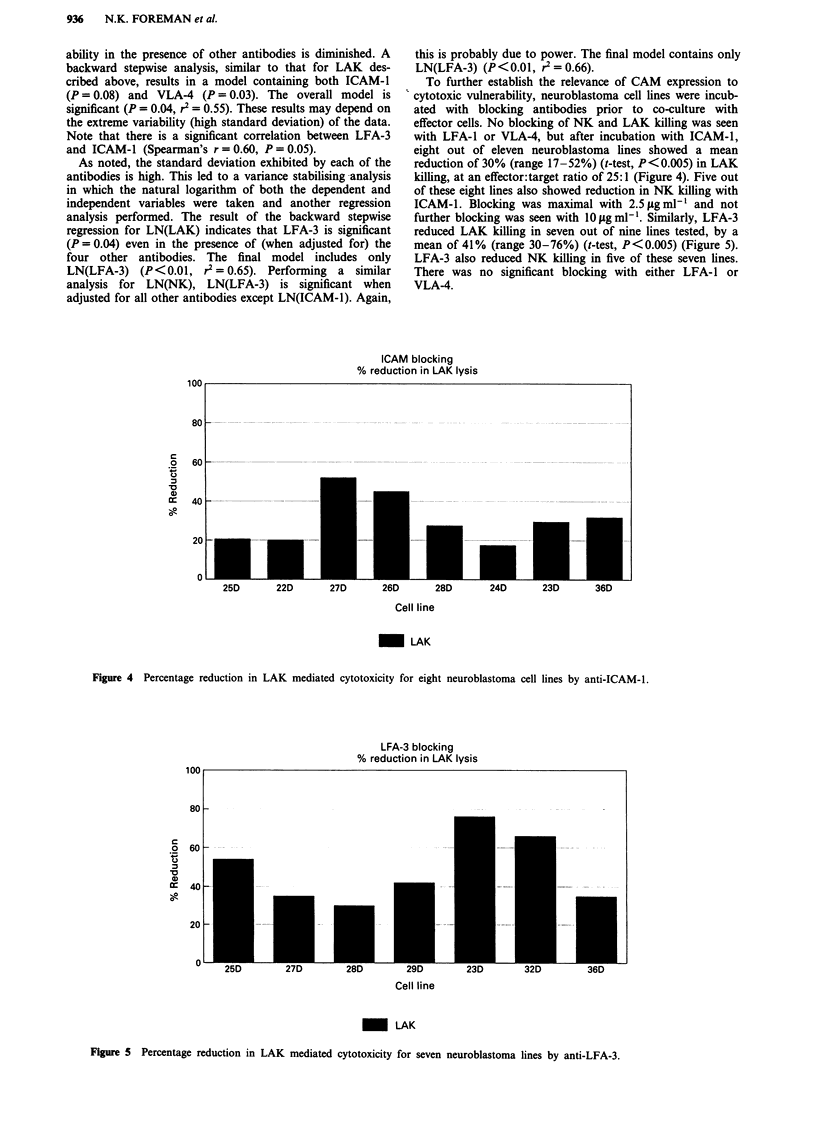
Widely disseminated neuroblastoma in children older than infancy remains a very poor prognosis disease. Even the introduction of marrow ablative chemotherapy with autologous rescue has not significantly improved the outlook for these children, presumably because of a failure to eradicate minimal residual disease. One additional approach which may hold promise is the use of immunomodulation with cytokines such as IL2 in the setting of minimal residual disease (MDR), for example after intensive chemotherapy and ABMT. However, considerable variability in the susceptibility of neuroblastoma cells to natural killer (NK) and lymphokine-activated (LAK) killing has been observed, and it is presently unclear how NK and LAK cells recognise neuroblastoma cells. In this paper we examine expression of cell adhesion molecules on neuroblastoma to determine which of these modify interaction with NK and LAK cells. We find that LFA-3 (CD58), the ligand for CD2 is of predominant importance in predicting susceptibility of neuroblastoma to the cytotoxic actions of NK and LAK cells, while expression of ICAM-1 (CD54) may also modify susceptibility. These findings were confirmed by blocking experiments in which co-culture of target cells with ICAM-1 and LFA-3 reduced LAK and NK cytotoxicity. Study of the immunophenotypic features of each patient's neuroblastoma cells before induction of MRD may be valuable in determining the likely effect of IL2 in predicting disease reactivation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. R., Coccia P. F. Is more better? Dose intensity in neuroblastoma. J Clin Oncol. 1991 Jun;9(6):902–904. doi: 10.1200/JCO.1991.9.6.902. [DOI] [PubMed] [Google Scholar]
- Anichini A., Mortarini R., Supino R., Parmiani G. Human melanoma cells with high susceptibility to cell-mediated lysis can be identified on the basis of ICAM-1 phenotype, VLA profile and invasive ability. Int J Cancer. 1990 Sep 15;46(3):508–515. doi: 10.1002/ijc.2910460330. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Fong C. T., Morita M., Griffith R., Hayes F. A., Seeger R. C. Molecular analysis and clinical significance of N-myc amplification and chromosome 1p monosomy in human neuroblastomas. Prog Clin Biol Res. 1988;271:3–15. [PubMed] [Google Scholar]
- Campana D., Coustan-Smith E., Janossy G. The immunologic detection of minimal residual disease in acute leukemia. Blood. 1990 Jul 1;76(1):163–171. [PubMed] [Google Scholar]
- D'Angio G. J., Evans A. E., Koop C. E. Special pattern of widespread neuroblastoma with a favourable prognosis. Lancet. 1971 May 22;1(7708):1046–1049. doi: 10.1016/s0140-6736(71)91606-0. [DOI] [PubMed] [Google Scholar]
- Duan D. S., Farmer D., Rayner A. A., Sadee W. Cytotoxicity of lymphokine-activated killer cells against human neuroblastoma cells: modulation by neuroblast differentiation. Med Pediatr Oncol. 1990;18(4):339–344. doi: 10.1002/mpo.2950180418. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Sanders M. E., Shaw S., Springer T. A. Purified lymphocyte function-associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J Exp Med. 1987 Mar 1;165(3):677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. E., Baum E., Chard R. Do infants with stage IV-S neuroblastoma need treatment? Arch Dis Child. 1981 Apr;56(4):271–274. doi: 10.1136/adc.56.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrot M. C., Combaret V., Goillot E., Tabone E., Bouffet E., Dolbeau D., Bouvier R., Coze C., Michon J., Philip T. Expression of leucocyte adhesion molecules on 66 clinical neuroblastoma specimens. Int J Cancer. 1991 Jun 19;48(4):502–510. doi: 10.1002/ijc.2910480405. [DOI] [PubMed] [Google Scholar]
- Gross N., Beck D., Favre S. In vitro modulation and relationship between N-myc and HLA class I RNA steady-state levels in human neuroblastoma cells. Cancer Res. 1990 Dec 1;50(23):7532–7536. [PubMed] [Google Scholar]
- Gross N., Carrel S., Beck D., Favre S. Cell adhesion molecules expression and modulation on human neuroblastoma cells. Prog Clin Biol Res. 1991;366:293–299. [PubMed] [Google Scholar]
- Handgretinger R., Bruchelt G., Kimmig A., Dopfer R., Niethammer D., Treuner J. In vitro induction of lymphokine-activated killer (LAK) activity in patients with neuroblastoma. Pediatr Hematol Oncol. 1989;6(4):307–317. doi: 10.3109/08880018909034302. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987 Feb 27;48(4):681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Look A. T., Hayes F. A., Shuster J. J., Douglass E. C., Castleberry R. P., Bowman L. C., Smith E. I., Brodeur G. M. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991 Apr;9(4):581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- Main E. K., Lampson L. A., Hart M. K., Kornbluth J., Wilson D. B. Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol. 1985 Jul;135(1):242–246. [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Shaw S. The CD2-LFA-3 and LFA-1-ICAM pathways: relevance to T-cell recognition. Immunol Today. 1989 Dec;10(12):417–422. doi: 10.1016/0167-5699(89)90039-X. [DOI] [PubMed] [Google Scholar]
- Nasr S., McKolanis J., Pais R., Findley H., Hnath R., Waldrep K., Ragab A. H. A phase I study of interleukin-2 in children with cancer and evaluation of clinical and immunologic status during therapy. A Pediatric Oncology Group Study. Cancer. 1989 Aug 15;64(4):783–788. doi: 10.1002/1097-0142(19890815)64:4<783::aid-cncr2820640402>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Negrier S., Michon J., Floret D., Bouffet E., Gentet J. C., Philip I., Cochat P., Stamm D., Costil J., Gaspard M. Interleukin-2 and lymphokine-activated killer cells in 15 children with advanced metastatic neuroblastoma. J Clin Oncol. 1991 Aug;9(8):1363–1370. doi: 10.1200/JCO.1991.9.8.1363. [DOI] [PubMed] [Google Scholar]
- Oblakowski P., Bello-Fernandez C., Reittie J. E., Heslop H. E., Galatowicz G., Veys P., Wilkes S., Prentice H. G., Hazlehurst G., Hoffbrand A. V. Possible mechanism of selective killing of myeloid leukemic blast cells by lymphokine-activated killer cells. Blood. 1991 May 1;77(9):1996–2001. [PubMed] [Google Scholar]
- Pole J. G., Casper J., Elfenbein G., Gee A., Gross S., Janssen W., Koch P., Marcus R., Pick T., Shuster J. High-dose chemoradiotherapy supported by marrow infusions for advanced neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991 Jan;9(1):152–158. doi: 10.1200/JCO.1991.9.1.152. [DOI] [PubMed] [Google Scholar]
- Pritchard J., McElwain T. J., Graham-Pole J. High-dose melphalan with autologous marrow for treatment of advanced neuroblastoma. Br J Cancer. 1982 Jan;45(1):86–94. doi: 10.1038/bjc.1982.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reittie J. E., Gottlieb D., Heslop H. E., Leger O., Drexler H. G., Hazlehurst G., Hoffbrand A. V., Prentice H. G., Brenner M. K. Endogenously generated activated killer cells circulate after autologous and allogeneic marrow transplantation but not after chemotherapy. Blood. 1989 Apr;73(5):1351–1358. [PubMed] [Google Scholar]
- Reynolds J. V., Shou J., Choi H., Sigal R., Ziegler M. M., Daly J. M. The influence of natural killer cells in neuroblastoma. Arch Surg. 1989 Feb;124(2):235–239. doi: 10.1001/archsurg.1989.01410020109018. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sussman E. H., Luce G. E., Cossman J., Shaw S. Molecular pathways of adhesion in spontaneous rosetting of T-lymphocytes to the Hodgkin's cell line L428. Cancer Res. 1988 Jan 1;48(1):37–40. [PubMed] [Google Scholar]
- Shuster J. J., Cantor A. B., McWilliams N., Pole J. G., Castleberry R. P., Marcus R., Pick T., Smith E. I., Hayes F. A. The prognostic significance of autologous bone marrow transplant in advanced neuroblastoma. J Clin Oncol. 1991 Jun;9(6):1045–1049. doi: 10.1200/JCO.1991.9.6.1045. [DOI] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]


