Abstract
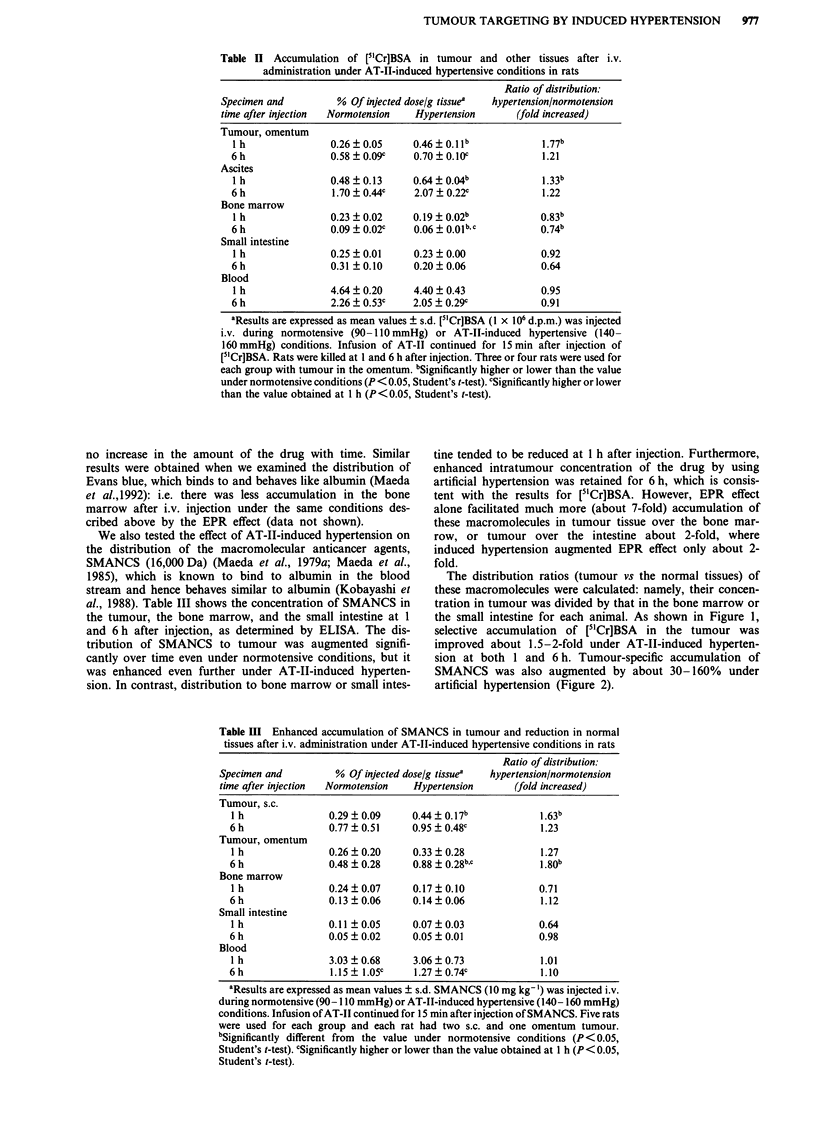
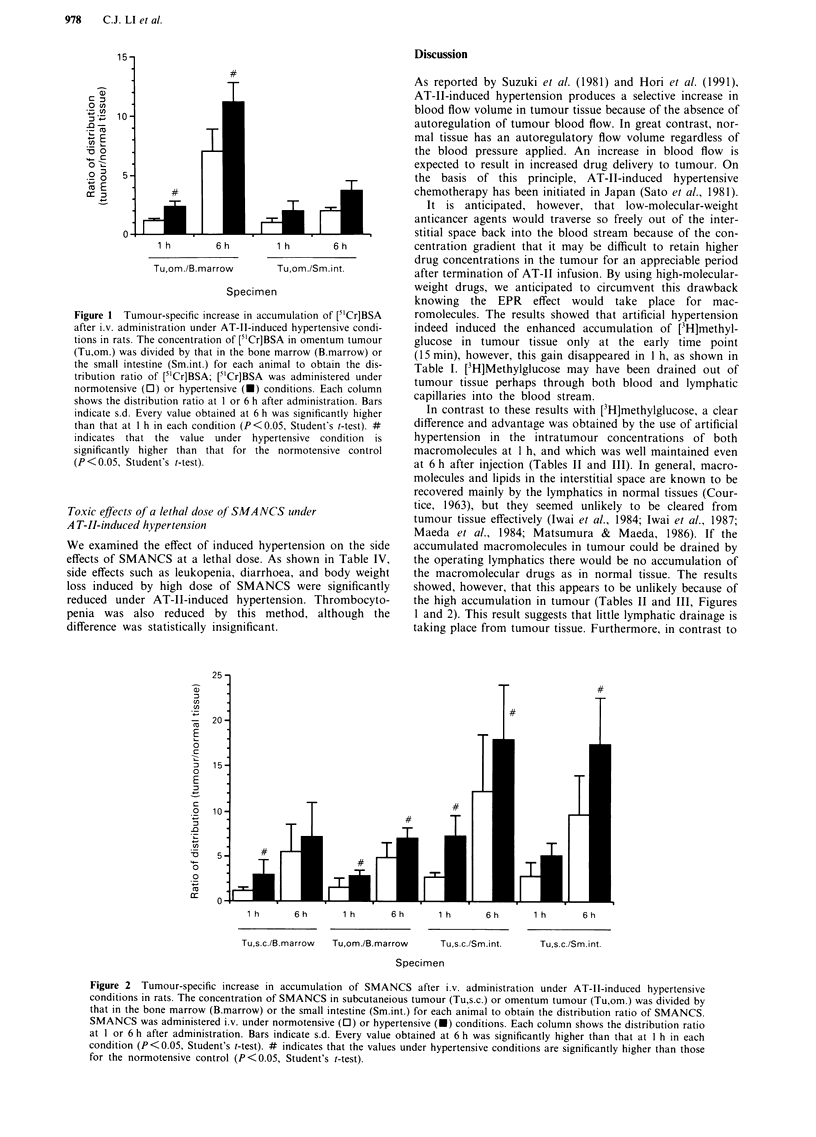
Effects of angiotensin II (AT-II)-induced hypertension on the distribution of macromolecules to Walker carcinoma and to bone marrow of SMANCS [poly(styrene-co-maleic-acid)-neocarzinostatin conjugate] were investigated in rats. AT-II-induced hypertension from about 100 to 150 mmHg significantly increased the accumulation of the macromolecular drug SMANCS and 51Cr-labelled bovine serum albumin ([51Cr]BSA), representatives of macromolecular drugs, in tumour tissue. At 1 h after i.v. administration, intratumour concentrations of [51Cr]BSA and SMANCS were elevated by 1.2-1.8-fold. The higher drug accumulation in the tumour that was produced by the artificial hypertension was retained even 6 h after administration. This observation indicates an additive effect to that under normotensive conditions where intratumour macromolecular drug concentrations increase steadily during this period. Furthermore, distributions of these drugs in the bone marrow and the small intestine decreased during artificial hypertension to 60-80% of those in the normotensive state. Therefore, the drug concentration ratios of tumour/bone marrow and tumour/small intestine were increased by 1.8-2.4-fold. A decreased distribution of SMANCS to normal tissues under hypertensive conditions was also confirmed by the significant reduction of its toxicity e.g. leukopenia, diarrhoea, and body weight loss, even at a lethal dose. On the contrary, [3H]methylglucose showed no remarkable difference in tumour or bone marrow accumulation under this hypertensive condition. These results show the advantages of macromolecules over small molecules for AT-II-induced hypertension chemotherapy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cope D. A., Dewhirst M. W., Friedman H. S., Bigner D. D., Zalutsky M. R. Enhanced delivery of a monoclonal antibody F(ab')2 fragment to subcutaneous human glioma xenografts using local hyperthermia. Cancer Res. 1990 Mar 15;50(6):1803–1809. [PubMed] [Google Scholar]
- Dvorak H. F., Senger D. R., Dvorak A. M., Harvey V. S., McDonagh J. Regulation of extravascular coagulation by microvascular permeability. Science. 1985 Mar 1;227(4690):1059–1061. doi: 10.1126/science.3975602. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Hnatowich D. J., Layne W. W., Childs R. L. The preparation and labeling of DTPA-coupled albumin. Int J Appl Radiat Isot. 1982 May;33(5):327–332. doi: 10.1016/0020-708x(82)90144-2. [DOI] [PubMed] [Google Scholar]
- Hori K., Suzuki M., Abe I., Saito S., Sato H. A micro-occlusion technique for measurement of the microvascular pressure in tumor and subcutis. Gan. 1983 Feb;74(1):122–127. [PubMed] [Google Scholar]
- Hori K., Suzuki M., Abe I., Saito S., Sato H. Increase in tumor vascular area due to increased blood flow by angiotensin II in rats. J Natl Cancer Inst. 1985 Feb;74(2):453–459. [PubMed] [Google Scholar]
- Hori K., Suzuki M., Tanda S., Saito S., Shinozaki M., Zhang Q. H. Fluctuations in tumor blood flow under normotension and the effect of angiotensin II-induced hypertension. Jpn J Cancer Res. 1991 Nov;82(11):1309–1316. doi: 10.1111/j.1349-7006.1991.tb01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai K., Maeda H., Konno T., Matsumura Y., Yamashita R., Yamasaki K., Hirayama S., Miyauchi Y. Tumor targeting by arterial administration of lipids: rabbit model with VX2 carcinoma in the liver. Anticancer Res. 1987 May-Jun;7(3 Pt B):321–327. [PubMed] [Google Scholar]
- Iwai K., Maeda H., Konno T. Use of oily contrast medium for selective drug targeting to tumor: enhanced therapeutic effect and X-ray image. Cancer Res. 1984 May;44(5):2115–2121. [PubMed] [Google Scholar]
- Jain R. K. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990 Feb 1;50(3 Suppl):814s–819s. [PubMed] [Google Scholar]
- Kalofonos H., Rowlinson G., Epenetos A. A. Enhancement of monoclonal antibody uptake in human colon tumor xenografts following irradiation. Cancer Res. 1990 Jan 1;50(1):159–163. [PubMed] [Google Scholar]
- Khaw B. A., Fallon F. T., Strauss H. W., Haber E. Myocardial infarct imaging of antibodies to canine cardiac myosin with indium-111-diethylenetriamine pentaacetic acid. Science. 1980 Jul 11;209(4453):295–297. doi: 10.1126/science.7384803. [DOI] [PubMed] [Google Scholar]
- Konno T., Maeda H., Iwai K., Maki S., Tashiro S., Uchida M., Miyauchi Y. Selective targeting of anti-cancer drug and simultaneous image enhancement in solid tumors by arterially administered lipid contrast medium. Cancer. 1984 Dec 1;54(11):2367–2374. doi: 10.1002/1097-0142(19841201)54:11<2367::aid-cncr2820541111>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Konno T., Maeda H., Iwai K., Tashiro S., Maki S., Morinaga T., Mochinaga M., Hiraoka T., Yokoyama I. Effect of arterial administration of high-molecular-weight anticancer agent SMANCS with lipid lymphographic agent on hepatoma: a preliminary report. Eur J Cancer Clin Oncol. 1983 Aug;19(8):1053–1065. doi: 10.1016/0277-5379(83)90028-7. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T., Aoki K., Taniguchi S., Hasuda K., Baba T. Efficacy of two-route chemotherapy using cis-diamminedichloroplatinum(II) and its antidote, sodium thiosulfate, in combination with angiotensin II in a rat limb tumor. Cancer Res. 1987 Jul 15;47(14):3618–3623. [PubMed] [Google Scholar]
- Maeda H., Matsumura Y., Kato H. Purification and identification of [hydroxyprolyl3]bradykinin in ascitic fluid from a patient with gastric cancer. J Biol Chem. 1988 Nov 5;263(31):16051–16054. [PubMed] [Google Scholar]
- Maeda H., Matsumura Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit Rev Ther Drug Carrier Syst. 1989;6(3):193–210. [PubMed] [Google Scholar]
- Maeda H., Seymour L. W., Miyamoto Y. Conjugates of anticancer agents and polymers: advantages of macromolecular therapeutics in vivo. Bioconjug Chem. 1992 Sep-Oct;3(5):351–362. doi: 10.1021/bc00017a001. [DOI] [PubMed] [Google Scholar]
- Maeda H., Takeshita J., Kanamaru R. A lipophilic derivative of neocarzinostatin. A polymer conjugation of an antitumor protein antibiotic. Int J Pept Protein Res. 1979 Aug;14(2):81–87. doi: 10.1111/j.1399-3011.1979.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Maeda H., Takeshita J., Kanamaru R., Sato H., Khatoh J., Sato H. Antimetastatic and antitumor activity of a derivative of neocarzinostatin: an organic solvent- and water-soluble polymer-conjugated protein. Gan. 1979 Oct;70(5):601–606. [PubMed] [Google Scholar]
- Maeda H., Ueda M., Morinaga T., Matsumoto T. Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: pronounced improvements in pharmacological properties. J Med Chem. 1985 Apr;28(4):455–461. doi: 10.1021/jm00382a012. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Kimura M., Yamamoto T., Maeda H. Involvement of the kinin-generating cascade in enhanced vascular permeability in tumor tissue. Jpn J Cancer Res. 1988 Dec;79(12):1327–1334. doi: 10.1111/j.1349-7006.1988.tb01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986 Dec;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- Matsumura Y., Maeda H., Kato H. Degradation pathway of kinins in tumor ascites and inhibition by kininase inhibitors: analysis by HPLC. Agents Actions. 1990 Mar;29(3-4):172–180. doi: 10.1007/BF01966443. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Maruo K., Kimura M., Yamamoto T., Konno T., Maeda H. Kinin-generating cascade in advanced cancer patients and in vitro study. Jpn J Cancer Res. 1991 Jun;82(6):732–741. doi: 10.1111/j.1349-7006.1991.tb01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi A., Takahashi T., Yamaguchi T., Kitamura K., Noguchi A., Tsurumi H., Takashina K., Maeda H. Enhanced tumor localization of monoclonal antibody by treatment with kininase II inhibitor and angiotensin II. Jpn J Cancer Res. 1992 Mar;83(3):240–243. doi: 10.1111/j.1349-7006.1992.tb00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Krauer K. G., McKenzie I. F., Pietersz G. A. Effect of tumor necrosis factor on the antitumor efficacy and toxicity of aminopterin-monoclonal antibody conjugates: parameters for optimization of therapy. Cancer Res. 1990 Sep 15;50(18):6028–6033. [PubMed] [Google Scholar]
- Sato H., Sato K., Sato Y., Asamura M., Kanamaru R., Sugiyama Z., Kitahara T., Mimata Y., Wakui A., Suzuki M. Induced hypertension chemotherapy of cancer patients by selective enhancement of drug delivery to tumor tissue with angiotensin II. Sci Rep Res Inst Tohoku Univ Med. 1981 Dec;28(1-4):32–44. [PubMed] [Google Scholar]
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Skinner S. A., Tutton P. J., O'Brien P. E. Microvascular architecture of experimental colon tumors in the rat. Cancer Res. 1990 Apr 15;50(8):2411–2417. [PubMed] [Google Scholar]
- Smyth M. J., Pietersz G. A., McKenzie I. F. Use of vasoactive agents to increase tumor perfusion and the antitumor efficacy of drug-monoclonal antibody conjugates. J Natl Cancer Inst. 1987 Dec;79(6):1367–1373. [PubMed] [Google Scholar]
- Suzuki M., Hori K., Abe I., Saito S., Sato H. A new approach to cancer chemotherapy: selective enhancement of tumor blood flow with angiotensin II. J Natl Cancer Inst. 1981 Sep;67(3):663–669. [PubMed] [Google Scholar]
- Suzuki M., Takahashi T., Sato T. Medial regression and its functional significance in tumor-supplying host arteries. A morphometric study of hepatic arteries in human livers with hepatocellular carcinoma. Cancer. 1987 Feb 1;59(3):444–450. doi: 10.1002/1097-0142(19870201)59:3<444::aid-cncr2820590316>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Takeshita J., Maeda H., Kanamaru R. In vitro mode of action, pharmacokinetics, and organ specificity of poly (maleic acid-styrene)-conjugated neocarzinostatin, SMANCS. Gan. 1982 Apr;73(2):278–284. [PubMed] [Google Scholar]
- Wakui A., Sato H. [Clinical studies on induced hypertension chemotherapy based on functional characteristics of microcirculation of tumor vessels]. Gan To Kagaku Ryoho. 1984 Mar;11(3 Pt 2):741–749. [PubMed] [Google Scholar]



