Abstract
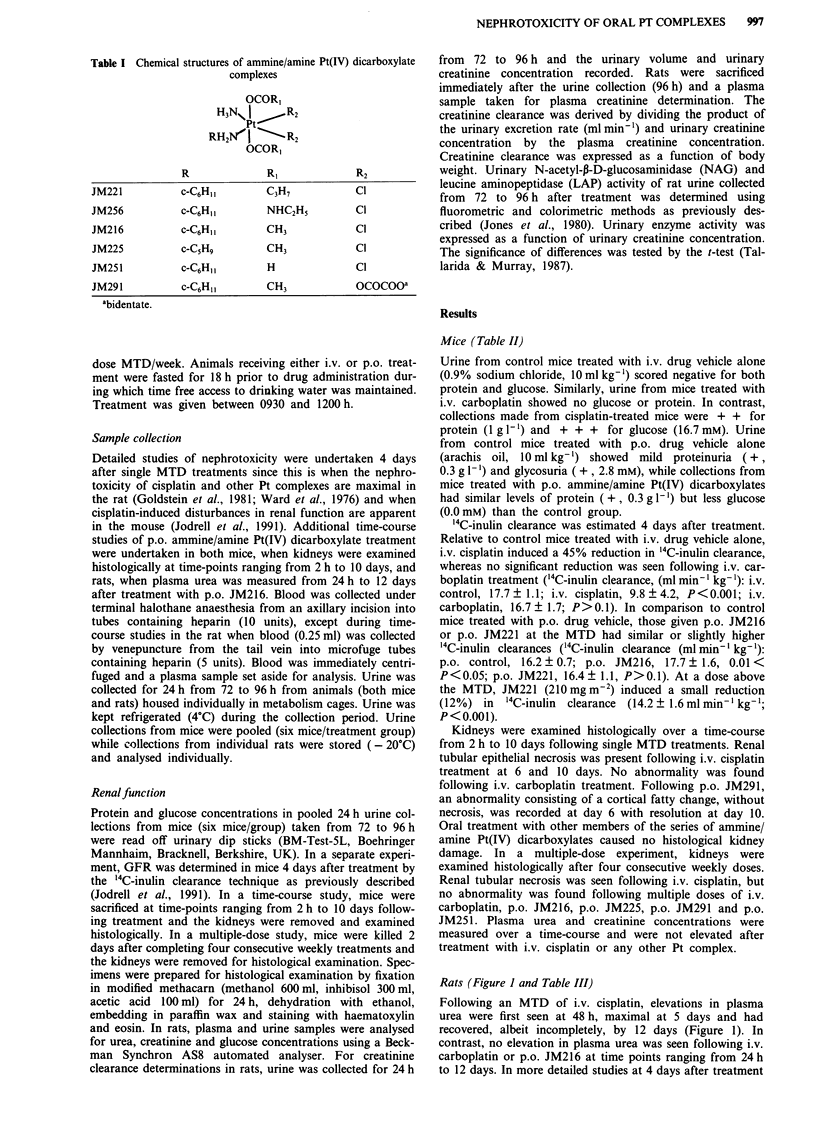
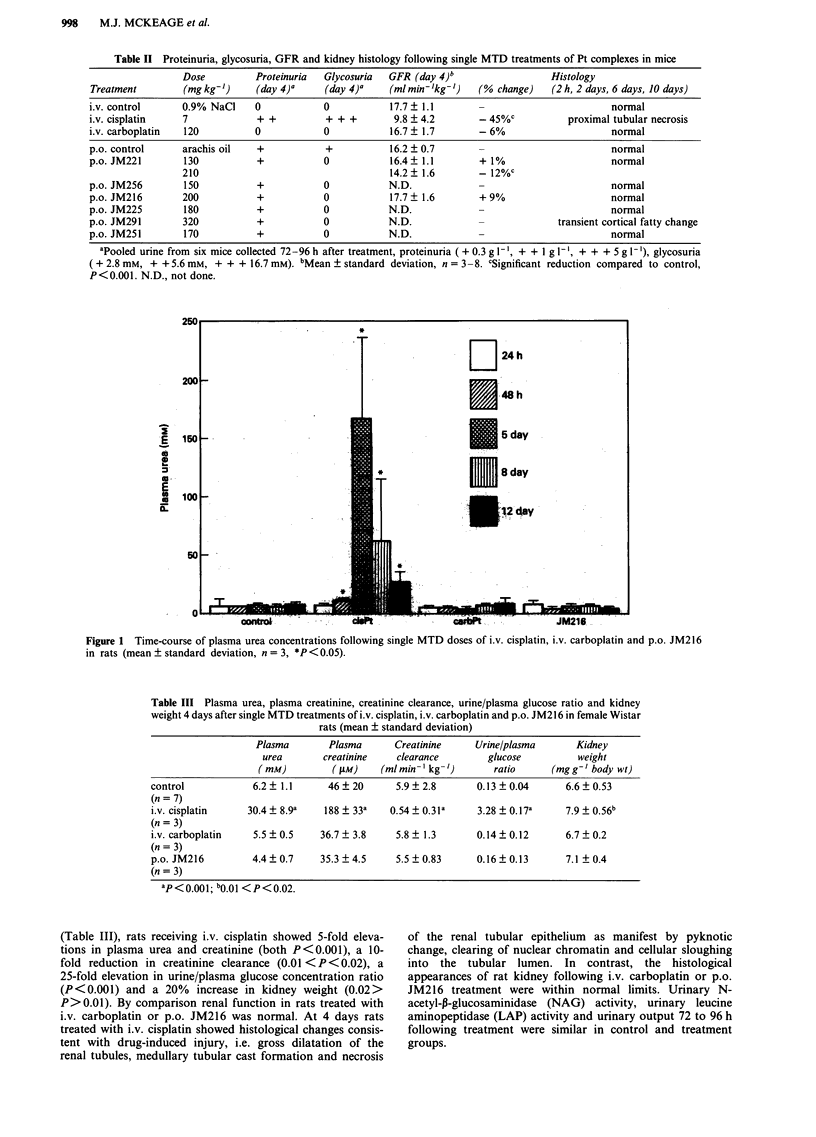
The comparative nephrotoxicity of i.v. cisplatin, i.v. carboplatin and six p.o. ammine/amine Pt(IV) dicarboxylates was studied in rodents following single MTD treatments. In mice, i.v. cisplatin caused proteinuria (1 g l-1), glycosuria (16.7 mM) and decreased GFR at 4 days, and histological kidney damage with onset at 6 days. In contrast, mice treated with i.v. carboplatin or p.o. ammine/amine Pt(IV) dicarboxylates had urinary glucose, urinary protein, GFR and kidney histology within the control range. In rats, i.v. cisplatin caused 5-fold elevations in plasma creatinine (188 +/- 33 microM) and urea (30.4 +/- 8.9 mM), a 10-fold fall in creatinine clearance (0.54 +/- 0.31 ml min-1 kg-1), a 25-fold elevation in urine/plasma glucose concentration ratio (3.28 +/- 0.17), a 20% increase in kidney weight (7.9 +/- 0.56 mg gm-1 body weight) and extensive histological damage 4 days after treatment. In contrast, i.v. carboplatin and p.o. JM216 (the lead compound of this series) caused neither abnormalities in renal function nor histological damage in rats. The nephrotoxicity of single MTD treatments of p.o. ammine/amine Pt(IV) dicarboxylate complexes appears less than i.v. cisplatin and comparable to i.v. carboplatin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Sarraf M., Fletcher W., Oishi N., Pugh R., Hewlett J. S., Balducci L., McCracken J., Padilla F. Cisplatin hydration with and without mannitol diuresis in refractory disseminated malignant melanoma: a southwest oncology group study. Cancer Treat Rep. 1982 Jan;66(1):31–35. [PubMed] [Google Scholar]
- Calvert A. H., Harland S. J., Newell D. R., Siddik Z. H., Jones A. C., McElwain T. J., Raju S., Wiltshaw E., Smith I. E., Baker J. M. Early clinical studies with cis-diammine-1,1-cyclobutane dicarboxylate platinum II. Cancer Chemother Pharmacol. 1982;9(3):140–147. doi: 10.1007/BF00257742. [DOI] [PubMed] [Google Scholar]
- Daugaard G., Abildgaard U., Holstein-Rathlou N. H., Bruunshuus I., Bucher D., Leyssac P. P. Renal tubular function in patients treated with high-dose cisplatin. Clin Pharmacol Ther. 1988 Aug;44(2):164–172. doi: 10.1038/clpt.1988.132. [DOI] [PubMed] [Google Scholar]
- Daugaard G., Rossing N., Rørth M. Effects of cisplatin on different measures of glomerular function in the human kidney with special emphasis on high-dose. Cancer Chemother Pharmacol. 1988;21(2):163–167. doi: 10.1007/BF00257365. [DOI] [PubMed] [Google Scholar]
- DeConti R. C., Toftness B. R., Lange R. C., Creasey W. A. Clinical and pharmacological studies with cis-diamminedichloroplatinum (II). Cancer Res. 1973 Jun;33(6):1310–1315. [PubMed] [Google Scholar]
- Goldstein R. S., Noordewier B., Bond J. T., Hook J. B., Mayor G. H. cis-Dichlorodiammineplatinum nephrotoxicity: time course and dose response of renal functional impairment. Toxicol Appl Pharmacol. 1981 Sep 15;60(2):163–175. doi: 10.1016/0041-008x(91)90220-9. [DOI] [PubMed] [Google Scholar]
- Gonzales-Vitale J. C., Hayes D. M., Cvitkovic E., Sternberg S. S. The renal pathology in clinical trials of cis-platinum (II) diamminedichloride. Cancer. 1977 Apr;39(4):1362–1371. doi: 10.1002/1097-0142(197704)39:4<1362::aid-cncr2820390403>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Gore M. E., Calvert A. H., Smith L. E. High dose carboplatin in the treatment of lung cancer and mesothelioma: a phase I dose escalation study. Eur J Cancer Clin Oncol. 1987 Sep;23(9):1391–1397. doi: 10.1016/0277-5379(87)90125-8. [DOI] [PubMed] [Google Scholar]
- Jodrell D. I., Newell D. R., Morgan S. E., Clinton S., Bensted J. P., Hughes L. R., Calvert A. H. The renal effects of N10-propargyl-5,8-dideazafolic acid (CB3717) and a non-nephrotoxic analogue ICI D1694, in mice. Br J Cancer. 1991 Nov;64(5):833–838. doi: 10.1038/bjc.1991.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. R., Bhalla R. B., Mladek J., Kaleya R. N., Gralla R. J., Alcock N. W., Schwartz M. K., Young C. W., Reidenberg M. M. Comparison of methods of evaluating nephrotoxicity of cis-platinum. Clin Pharmacol Ther. 1980 Apr;27(4):557–562. doi: 10.1038/clpt.1980.79. [DOI] [PubMed] [Google Scholar]
- Kelland L. R., Murrer B. A., Abel G., Giandomenico C. M., Mistry P., Harrap K. R. Ammine/amine platinum(IV) dicarboxylates: a novel class of platinum complex exhibiting selective cytotoxicity to intrinsically cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 1992 Feb 15;52(4):822–828. [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Mason M. D., Nicholls J., Horwich A. The effect of carboplatin on renal function in patients with metastatic germ cell tumours. Br J Cancer. 1991 Apr;63(4):630–633. doi: 10.1038/bjc.1991.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossof A. H., Slayton R. E., Perlia C. P. Preliminary clinical experience with cis-diamminedichloroplatinum (II) (NSC 119875, CACP). Cancer. 1972 Dec;30(6):1451–1456. doi: 10.1002/1097-0142(197212)30:6<1451::aid-cncr2820300606>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sleijfer D. T., Smit E. F., Meijer S., Mulder N. H., Postmus P. E. Acute and cumulative effects of carboplatin on renal function. Br J Cancer. 1989 Jul;60(1):116–120. doi: 10.1038/bjc.1989.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Young D. M., Fauvie K. A., Wolpert M. K., Davis R., Guarino A. M. Comparative nephrotoxicity of platinum cancer chemotherapeutic agents. Cancer Treat Rep. 1976 Nov;60(11):1675–1678. [PubMed] [Google Scholar]



