Abstract
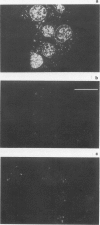
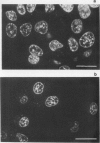
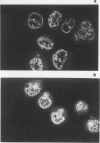
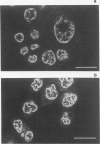
This study highlights the usefulness of laser scanning confocal microscopy in the examination of subcellular disposition of anthracyclines in tumour cell lines. The distribution of anthracycline compounds has been studied in two pairs of parental and multidrug resistant (MDR) cell lines. For the parental EMT6 mouse mammary tumour cell line EMT6/P treated with doxorubicin (DOX) the anthracycline fluorescence was shown to be predominantly nuclear but with some particulate cytoplasmic fluorescence and very low levels of plasma membrane staining. In the same experiments much fainter fluorescence was seen for the EMT6/AR1.0 MDR subline which hyperexpresses P-glycoprotein. The loss of nuclear fluorescence was comparatively greater than loss of cytoplasmic fluorescence. For the human large cell lung cancer line COR-L23/P cellular DOX disposition was markedly nuclear with nuclear membrane staining and diffuse cytoplasmic fluorescence. For the MDR line COR-L23/R, which lacks P-glycoprotein expression, DOX fluorescence was reduced in the nucleus compared with the parental line, but an intense area of perinuclear staining was seen consistent with localisation to the Golgi apparatus. The morpholinyl-substituted analogue MR-DOX achieved very similar subcellular distribution in both parental and MDR lines, consistent with its retention of activity in the latter. The presence of verapamil during anthracycline exposure increased the intensity of fluorescence in the MDR lines, particularly in the nucleus. Relatively little effect was seen in the parental lines. Confocal microscopy provides high resolution images of the subcellular distribution of anthracyclines in parent and MDR cell lines. Differences in drug disposition in various cell lines may provide insights into the mechanism of multidrug resistance and suggest strategies for its therapeutic circumvention.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrand M. A., Rhodes T., Center M. S., Twentyman P. R. Chemosensitisation and drug accumulation effects of cyclosporin A, PSC-833 and verapamil in human MDR large cell lung cancer cells expressing a 190k membrane protein distinct from P-glycoprotein. Eur J Cancer. 1993;29A(3):408–415. doi: 10.1016/0959-8049(93)90397-x. [DOI] [PubMed] [Google Scholar]
- Coley H. M., Twentyman P. R., Workman P. 9-Alkyl, morpholinyl anthracyclines in the circumvention of multidrug resistance. Eur J Cancer. 1990;26(6):665–667. doi: 10.1016/0277-5379(90)90112-7. [DOI] [PubMed] [Google Scholar]
- Coley H. M., Twentyman P. R., Workman P. Identification of anthracyclines and related agents that retain preferential activity over adriamycin in multidrug-resistant cell lines, and further resistance modification by verapamil and cyclosporin A. Cancer Chemother Pharmacol. 1989;24(5):284–290. doi: 10.1007/BF00304759. [DOI] [PubMed] [Google Scholar]
- Coley H. M., Twentyman P. R., Workman P. Improved cellular accumulation is characteristic of anthracyclines which retain high activity in multidrug resistant cell lines, alone or in combination with verapamil or cyclosporin A. Biochem Pharmacol. 1989 Dec 15;38(24):4467–4475. doi: 10.1016/0006-2952(89)90658-8. [DOI] [PubMed] [Google Scholar]
- Coley H. M., Workman P., Twentyman P. R. Retention of activity by selected anthracyclines in a multidrug resistant human large cell lung carcinoma line without P-glycoprotein hyperexpression. Br J Cancer. 1991 Mar;63(3):351–357. doi: 10.1038/bjc.1991.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietel M., Seidel A. Morphologic alterations in drug sensitive vs. drug resistant cells due to cytostatic application. Cancer Treat Rev. 1990 Dec;17 (Suppl A):3–10. doi: 10.1016/0305-7372(90)90010-d. [DOI] [PubMed] [Google Scholar]
- Egorin M. J., Clawson R. E., Cohen J. L., Ross L. A., Bachur N. R. Cytofluorescence localization of anthracycline antibiotics. Cancer Res. 1980 Dec;40(12):4669–4676. [PubMed] [Google Scholar]
- Egorin M. J., Clawson R. E., Ross L. A., Schlossberger N. M., Bachur N. R. Cellular accumulation and disposition of aclacinomycin A. Cancer Res. 1979 Nov;39(11):4396–4400. [PubMed] [Google Scholar]
- Egorin M. J., Hildebrand R. C., Cimino E. F., Bachur N. R. Cytofluorescence localization of adriamycin and daunorubicin. Cancer Res. 1974 Sep;34(9):2243–2245. [PubMed] [Google Scholar]
- Gervasoni J. E., Jr, Fields S. Z., Krishna S., Baker M. A., Rosado M., Thuraisamy K., Hindenburg A. A., Taub R. N. Subcellular distribution of daunorubicin in P-glycoprotein-positive and -negative drug-resistant cell lines using laser-assisted confocal microscopy. Cancer Res. 1991 Sep 15;51(18):4955–4963. [PubMed] [Google Scholar]
- Gigli M., Rasoanaivo T. W., Millot J. M., Jeannesson P., Rizzo V., Jardillier J. C., Arcamone F., Manfait M. Correlation between growth inhibition and intranuclear doxorubicin and 4'-deoxy-4'-iododoxorubicin quantitated in living K562 cells by microspectrofluorometry. Cancer Res. 1989 Feb 1;49(3):560–564. [PubMed] [Google Scholar]
- Hill B. T., Dennis L. Y., Li X. T., Whelan R. D. Identification of anthracycline analogues with enhanced cytotoxicity and lack of cross-resistance to adriamycin using a series of mammalian cell lines in vitro. Cancer Chemother Pharmacol. 1985;14(3):194–201. doi: 10.1007/BF00258115. [DOI] [PubMed] [Google Scholar]
- Hindenburg A. A., Gervasoni J. E., Jr, Krishna S., Stewart V. J., Rosado M., Lutzky J., Bhalla K., Baker M. A., Taub R. N. Intracellular distribution and pharmacokinetics of daunorubicin in anthracycline-sensitive and -resistant HL-60 cells. Cancer Res. 1989 Aug 15;49(16):4607–4614. [PubMed] [Google Scholar]
- Keizer H. G., Schuurhuis G. J., Broxterman H. J., Lankelma J., Schoonen W. G., van Rijn J., Pinedo H. M., Joenje H. Correlation of multidrug resistance with decreased drug accumulation, altered subcellular drug distribution, and increased P-glycoprotein expression in cultured SW-1573 human lung tumor cells. Cancer Res. 1989 Jun 1;49(11):2988–2993. [PubMed] [Google Scholar]
- Krishan A., Israel M., Modest E. J., Frei E., 3rd Differences in cellular uptake and cytofluorescence of adriamycin and N-trifluoroacetyladriamycin-14-valerate. Cancer Res. 1976 Jun;36(6):2108–2109. [PubMed] [Google Scholar]
- Lelong I. H., Guzikowski A. P., Haugland R. P., Pastan I., Gottesman M. M., Willingham M. C. Fluorescent verapamil derivative for monitoring activity of the multidrug transporter. Mol Pharmacol. 1991 Oct;40(4):490–494. [PubMed] [Google Scholar]
- Lothstein L., Sweatman T. W., Dockter M. E., Israel M. Resistance to N-benzyladriamycin-14-valerate in mouse J774.2 cells: P-glycoprotein expression without reduced N-benzyladriamycin-14-valerate accumulation. Cancer Res. 1992 Jun 15;52(12):3409–3417. [PubMed] [Google Scholar]
- Marquardt D., Center M. S. Drug transport mechanisms in HL60 cells isolated for resistance to adriamycin: evidence for nuclear drug accumulation and redistribution in resistant cells. Cancer Res. 1992 Jun 1;52(11):3157–3163. [PubMed] [Google Scholar]
- Reeve J. G., Rabbitts P. H., Twentyman P. R. Non-P-glycoprotein-mediated multidrug resistance with reduced EGF receptor expression in a human large cell lung cancer cell line. Br J Cancer. 1990 Jun;61(6):851–855. doi: 10.1038/bjc.1990.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., Cervantes A., van Heijningen T. H., de Lange J. H., Baak J. P., Pinedo H. M., Lankelma J. Quantitative determination of factors contributing to doxorubicin resistance in multidrug-resistant cells. J Natl Cancer Inst. 1989 Dec 20;81(24):1887–1892. doi: 10.1093/jnci/81.24.1887. [DOI] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., de Lange J. H., Pinedo H. M., van Heijningen T. H., Kuiper C. M., Scheffer G. L., Scheper R. J., van Kalken C. K., Baak J. P. Early multidrug resistance, defined by changes in intracellular doxorubicin distribution, independent of P-glycoprotein. Br J Cancer. 1991 Nov;64(5):857–861. doi: 10.1038/bjc.1991.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. A., Westmacott D., Broadhurst M. J., Thomas G. J., Hall M. J. 9-alkyl anthracyclines. Absence of cross-resistance to adriamycin in human and murine cell cultures. Br J Cancer. 1986 May;53(5):595–600. doi: 10.1038/bjc.1986.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji Y., Fisher M. H., Periasamy A., Herman B., Juliano R. L. Verapamil and cyclosporin A modulate doxorubicin toxicity by distinct mechanisms. Cancer Lett. 1991 May 24;57(3):209–218. doi: 10.1016/0304-3835(91)90159-f. [DOI] [PubMed] [Google Scholar]
- Streeter D. G., Taylor D. L., Acton E. M., Peters J. H. Comparative cytotoxicities of various morpholinyl anthracyclines. Cancer Chemother Pharmacol. 1985;14(2):160–164. doi: 10.1007/BF00434357. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Kitatani Y., Yokota K., Tsukagoshi S., Sakurai Y. Effects of quinidine and related compounds on cytotoxicity and cellular accumulation of vincristine and adriamycin in drug-resistant tumor cells. Cancer Res. 1984 Oct;44(10):4303–4307. [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Wright K. A., Workman P., Broadhurst M. J., Martin J. A., Bleehen N. M. The in vitro effects and cross-resistance patterns of some novel anthracyclines. Br J Cancer. 1986 May;53(5):585–594. doi: 10.1038/bjc.1986.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R., Reeve J. G., Koch G., Wright K. A. Chemosensitisation by verapamil and cyclosporin A in mouse tumour cells expressing different levels of P-glycoprotein and CP22 (sorcin). Br J Cancer. 1990 Jul;62(1):89–95. doi: 10.1038/bjc.1990.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Amos W. B., Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987 Jul;105(1):41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Cornwell M. M., Cardarelli C. O., Gottesman M. M., Pastan I. Single cell analysis of daunomycin uptake and efflux in multidrug-resistant and -sensitive KB cells: effects of verapamil and other drugs. Cancer Res. 1986 Nov;46(11):5941–5946. [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]