Abstract
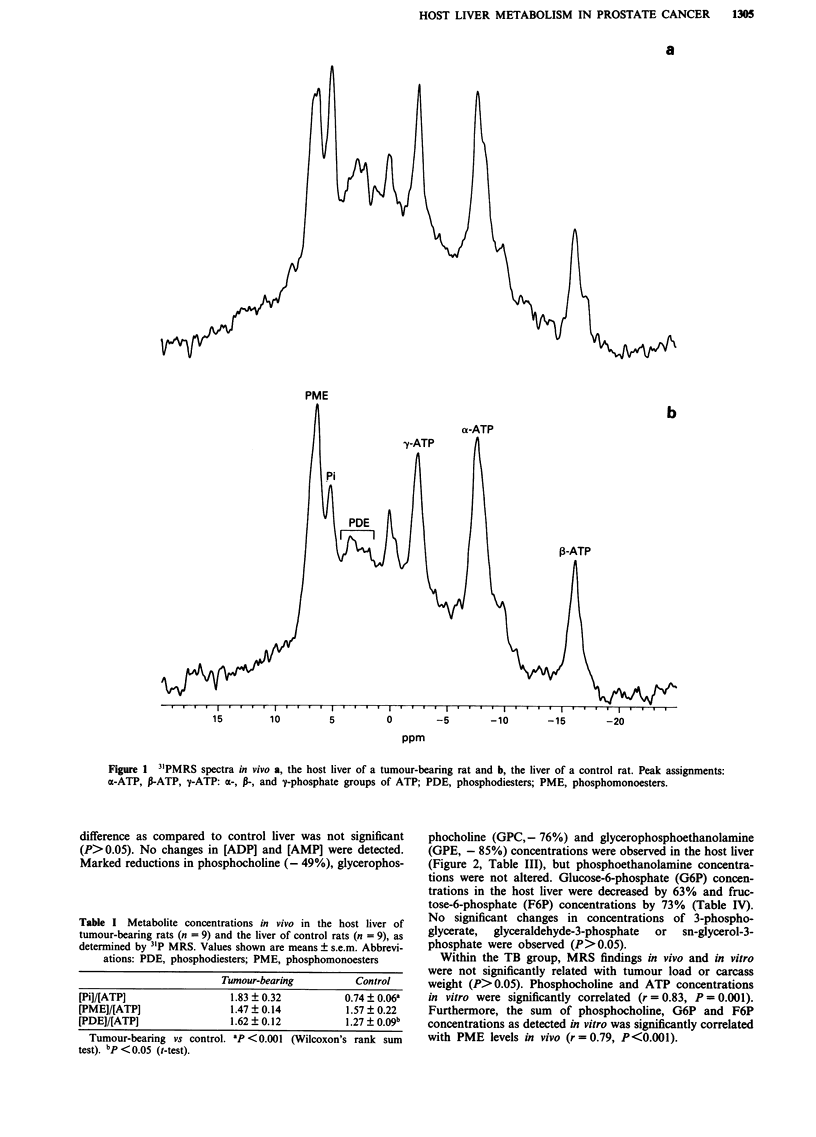
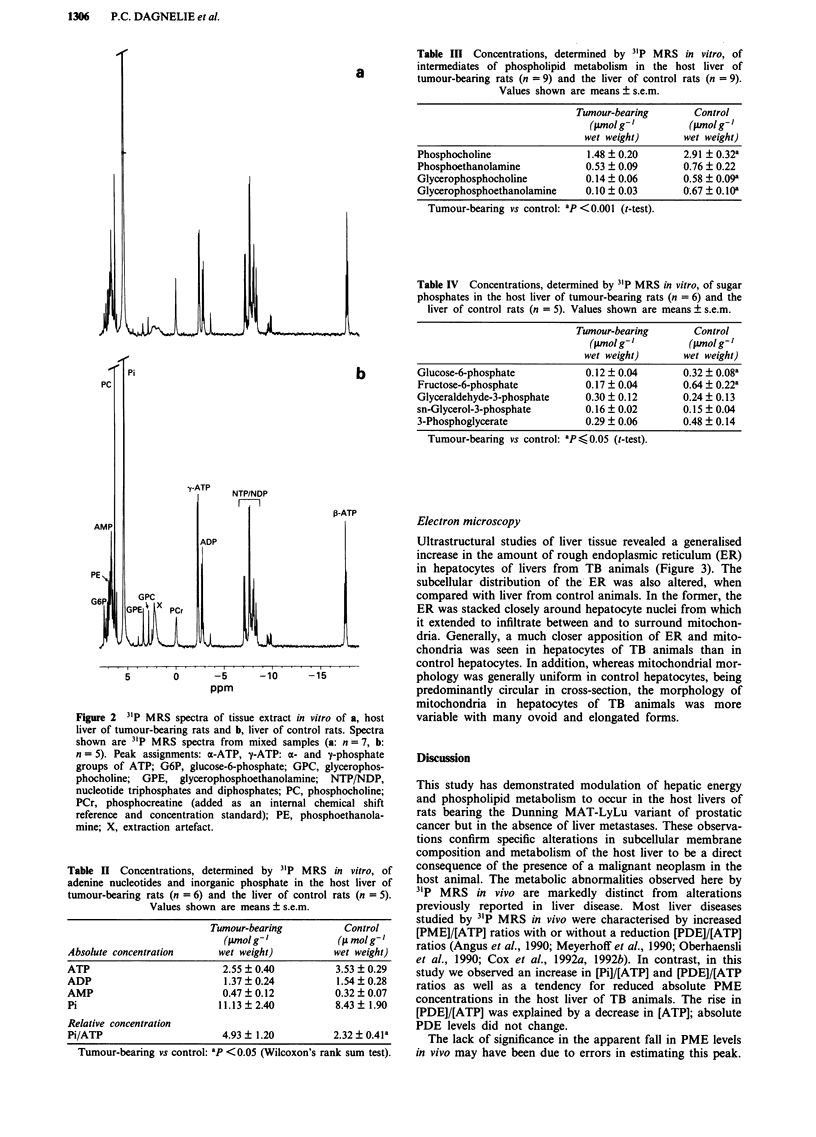
31P magnetic resonance spectroscopy (MRS) in vivo and in vitro was used to study modulation of host liver (HL) metabolism in rats bearing the MAT-LyLu variant of the Dunning prostate tumour. Animals were inoculated either with 10(6) or 10(7) MAT-LyLu cells, or with saline to serve as controls. Carcass weight in tumour-bearing (TB) animals decreased despite similar food and water intake in both groups. Absence of metastatic tumour cells from HL of all TB animals was confirmed by histological examination. Twenty-one days after inoculation, 31P MRS showed a 2.5-fold increase in [Pi]/[ATP] ratios in HL in vivo (P < 0.001) which was confirmed by 31P MRS of liver extracts in vitro (P < 0.005). Phosphodiester to ATP ratios were significantly increased (P < 0.05) in HL in vivo, but absolute PDE levels were similar in both groups. Phosphomonoester to ATP ratios did not change, although absolute phosphomonoester levels in HL were reduced by -41% (not significant). In HL extracts in vitro, sharp reductions in the levels of glucose-6-phosphate (P < 0.05), fructose-6-phosphate (P = 0.05), phosphocholine (P < 0.001), glycerophosphocholine (P < 0.001), and glycerophosphoethanolamine (P < 0.001) were observed. Electron microscopy revealed increased amounts and altered distribution of rough endoplasmic reticulum in HL. These findings show that experimental prostate cancer significantly affects hepatic phosphorylation status, phospholipid metabolism, and gluconeogenesis in the host animal, and demonstrate the value of combined MRS in vivo and in vitro in monitoring HL metabolism in cancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus P. W., Dixon R. M., Rajagopalan B., Ryley N. G., Simpson K. J., Peters T. J., Jewell D. P., Radda G. K. A study of patients with alcoholic liver disease by 31P nuclear magnetic resonance spectroscopy. Clin Sci (Lond) 1990 Jan;78(1):33–38. doi: 10.1042/cs0780033. [DOI] [PubMed] [Google Scholar]
- Argilés J. M., López-Soriano F. J. The energy state of tumor-bearing rats. J Biol Chem. 1991 Feb 15;266(5):2978–2982. [PubMed] [Google Scholar]
- Bashir I., Sikora K., Foster C. S. Cell-surface oligosaccharides expressed by phenotypically distinct sublines of the Dunning 3327 rat prostate cancer. Biochem Soc Trans. 1990 Oct;18(5):968–969. doi: 10.1042/bst0180968. [DOI] [PubMed] [Google Scholar]
- Bates T. E., Williams S. R., Busza A. L., Gadian D. G., Proctor E. A 31P nuclear magnetic resonance study in vivo of metabolic abnormalities in rats with acute liver failure. NMR Biomed. 1988 Apr;1(2):67–73. doi: 10.1002/nbm.1940010203. [DOI] [PubMed] [Google Scholar]
- Bates T. E., Williams S. R., Gadian D. G. Phosphodiesters in the liver: the effect of field strength on the 31P signal. Magn Reson Med. 1989 Oct;12(1):145–150. doi: 10.1002/mrm.1910120116. [DOI] [PubMed] [Google Scholar]
- Cox I. J., Bell J. D., Peden C. J., Iles R. A., Foster C. S., Watanapa P., Williamson R. C. In vivo and in vitro 31P magnetic resonance spectroscopy of focal hepatic malignancies. NMR Biomed. 1992 May-Jun;5(3):114–120. doi: 10.1002/nbm.1940050303. [DOI] [PubMed] [Google Scholar]
- Cox I. J., Menon D. K., Sargentoni J., Bryant D. J., Collins A. G., Coutts G. A., Iles R. A., Bell J. D., Benjamin I. S., Gilbey S. Phosphorus-31 magnetic resonance spectroscopy of the human liver using chemical shift imaging techniques. J Hepatol. 1992 Mar;14(2-3):265–275. doi: 10.1016/0168-8278(92)90169-p. [DOI] [PubMed] [Google Scholar]
- DUNNING W. F. PROSTATE CANCER IN THE RAT. Natl Cancer Inst Monogr. 1963 Oct;12:351–369. [PubMed] [Google Scholar]
- Daly P. F., Cohen J. S. Magnetic resonance spectroscopy of tumors and potential in vivo clinical applications: a review. Cancer Res. 1989 Feb 15;49(4):770–779. [PubMed] [Google Scholar]
- Desmoulin F., Canioni P., Masson S., Gérolami A., Cozzone P. J. Effect of ethanol on hepatic energy metabolism and intracellular pH in chronically ethanol-treated rats. A 31P NMR study of normoxic or hypoxic perfused liver. NMR Biomed. 1990 Jun;3(3):132–138. doi: 10.1002/nbm.1940030306. [DOI] [PubMed] [Google Scholar]
- Desmoulin F., Cozzone P. J., Canioni P. Phosphorus-31 nuclear-magnetic-resonance study of phosphorylated metabolites compartmentation, intracellular pH and phosphorylation state during normoxia, hypoxia and ethanol perfusion, in the perfused rat liver. Eur J Biochem. 1987 Jan 2;162(1):151–159. doi: 10.1111/j.1432-1033.1987.tb10555.x. [DOI] [PubMed] [Google Scholar]
- Effect of transplanted human ovarian cancer tissue on liver lipid metabolism of nude mice. Lipids. 1976 Feb;11(2):159–161. doi: 10.1007/BF02532668. [DOI] [PubMed] [Google Scholar]
- Foster C. S. Predictive factors in prostatic hyperplasia and neoplasia. Hum Pathol. 1990 Jun;21(6):575–577. doi: 10.1016/s0046-8177(96)90001-5. [DOI] [PubMed] [Google Scholar]
- Gutman A., Thilo E., Biran S. Enzymes of gluconeogenesis in tumor-bearing rats. Isr J Med Sci. 1969 Sep-Oct;5(5):998–1001. [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. The dedifferentiated pattern of enzymes in livers of tumor-bearing rats. Cancer Res. 1972 Sep;32(9):1826–1832. doi: 10.2172/4649739. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. The effect of lymphoma and other neoplasms on hepatic and plasma enzymes of the host rat. Cancer Res. 1977 Jan;37(1):231–238. [PubMed] [Google Scholar]
- Iles R. A., Stevens A. N., Griffiths J. R., Morris P. G. Phosphorylation status of liver by 31P-n.m.r. spectroscopy, and its implications for metabolic control. A comparison of 31P-n.m.r. spectroscopy (in vivo and in vitro) with chemical and enzymic determinations of ATP, ADP and Pi. Biochem J. 1985 Jul 1;229(1):141–151. doi: 10.1042/bj2290141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern K. A., Norton J. A. Cancer cachexia. JPEN J Parenter Enteral Nutr. 1988 May-Jun;12(3):286–298. doi: 10.1177/0148607188012003286. [DOI] [PubMed] [Google Scholar]
- Lawson D. H., Richmond A., Nixon D. W., Rudman D. Metabolic approaches to cancer cachexia. Annu Rev Nutr. 1982;2:277–301. doi: 10.1146/annurev.nu.02.070182.001425. [DOI] [PubMed] [Google Scholar]
- Ling M. F., Brauer M. In vitro 31P-NMR spectroscopic studies of rat liver subjected to chronic ethanol administration. Biochim Biophys Acta. 1990 Feb 19;1051(2):151–158. doi: 10.1016/0167-4889(90)90187-i. [DOI] [PubMed] [Google Scholar]
- Liu K. J., Henderson T. O., Kleps R. A., Reyes M. C., Nyhus L. M. Gluconeogenesis in the liver of tumor rats. J Surg Res. 1990 Aug;49(2):179–185. doi: 10.1016/0022-4804(90)90259-5. [DOI] [PubMed] [Google Scholar]
- Meyerhoff D. J., Karczmar G. S., Weiner M. W. Abnormalities of the liver evaluated by 31P MRS. Invest Radiol. 1989 Dec;24(12):980–984. doi: 10.1097/00004424-198912000-00012. [DOI] [PubMed] [Google Scholar]
- Murphy E. J., Rajagopalan B., Brindle K. M., Radda G. K. Phospholipid bilayer contribution to 31P NMR spectra in vivo. Magn Reson Med. 1989 Nov;12(2):282–289. doi: 10.1002/mrm.1910120218. [DOI] [PubMed] [Google Scholar]
- Nakazawa I., Yamagata S. Biochemical changes of the lipid in biopsied livers of patients with malignant neoplastic diseases. Tohoku J Exp Med. 1971 Feb;103(2):129–139. doi: 10.1620/tjem.103.129. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Vydelingum N. A., Brennan M. F. The reversal of increased gluconeogenesis in the tumor-bearing rat by tumor removal and food intake. Surgery. 1989 Aug;106(2):423–431. [PubMed] [Google Scholar]
- Oberhaensli R., Rajagopalan B., Galloway G. J., Taylor D. J., Radda G. K. Study of human liver disease with P-31 magnetic resonance spectroscopy. Gut. 1990 Apr;31(4):463–467. doi: 10.1136/gut.31.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Regulation of phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984 Jun 25;779(2):217–251. doi: 10.1016/0304-4157(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Podo F., Carpinelli G., Di Vito M., Giannini M., Proietti E., Fiers W., Gresser I., Belardelli F. Nuclear magnetic resonance analysis of tumor necrosis factor-induced alterations of phospholipid metabolites and pH in Friend leukemia cell tumors and fibrosarcomas in mice. Cancer Res. 1987 Dec 15;47(24 Pt 1):6481–6489. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radda G. K., Rajagopalan B., Taylor D. J. Biochemistry in vivo: an appraisal of clinical magnetic resonance spectroscopy. Magn Reson Q. 1989 Apr;5(2):122–151. [PubMed] [Google Scholar]
- Roh M. S., Ekman L. G., Jeevanandam M., Brennan M. F. Elevated energy expenditure in hepatocytes from tumor-bearing rats. J Surg Res. 1985 May;38(5):407–415. doi: 10.1016/0022-4804(85)90055-1. [DOI] [PubMed] [Google Scholar]
- Rossi-Fanelli F., Cascino A., Muscaritoli M. Abnormal substrate metabolism and nutritional strategies in cancer management. JPEN J Parenter Enteral Nutr. 1991 Nov-Dec;15(6):680–683. doi: 10.1177/0148607191015006680. [DOI] [PubMed] [Google Scholar]
- Schneeberger A. L., Thompson R. T., Driedger A. A., Finley R. J., Inculet R. I. Effect of cancer on the in vivo energy state of rat liver and skeletal muscle. Cancer Res. 1989 Mar 1;49(5):1160–1164. [PubMed] [Google Scholar]
- Shearer J., Caldwell M., Crosby L. O., Miller E., Buzby G. P., Mullen J. L. Tumor effects on gluconeogenesis in the isolated perfused rat liver. JPEN J Parenter Enteral Nutr. 1983 Mar-Apr;7(2):105–109. doi: 10.1177/0148607183007002105. [DOI] [PubMed] [Google Scholar]
- Smith T. A., Eccles S., Ormerod M. G., Tombs A. J., Titley J. C., Leach M. O. The phosphocholine and glycerophosphocholine content of an oestrogen-sensitive rat mammary tumour correlates strongly with growth rate. Br J Cancer. 1991 Nov;64(5):821–826. doi: 10.1038/bjc.1991.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternell M., Zachrisson H., Lundholm K. RNA polymerase activity and protein synthesis in mouse tumor-host liver compared to benign para-neoplastic reactions. Int J Cancer. 1988 Sep 15;42(3):464–469. doi: 10.1002/ijc.2910420326. [DOI] [PubMed] [Google Scholar]
- Tijburg L. B., Geelen M. J., van Golde L. M. Regulation of the biosynthesis of triacylglycerol, phosphatidylcholine and phosphatidylethanolamine in the liver. Biochim Biophys Acta. 1989 Jul 17;1004(1):1–19. doi: 10.1016/0005-2760(89)90206-3. [DOI] [PubMed] [Google Scholar]
- Wilkening J., Nowack J., Decker K. The dependence of glucose formation from lactate on the adenosine triphosphate content in the isolated perfused rat liver. Biochim Biophys Acta. 1975 Jun 12;392(2):299–309. doi: 10.1016/0304-4165(75)90011-2. [DOI] [PubMed] [Google Scholar]