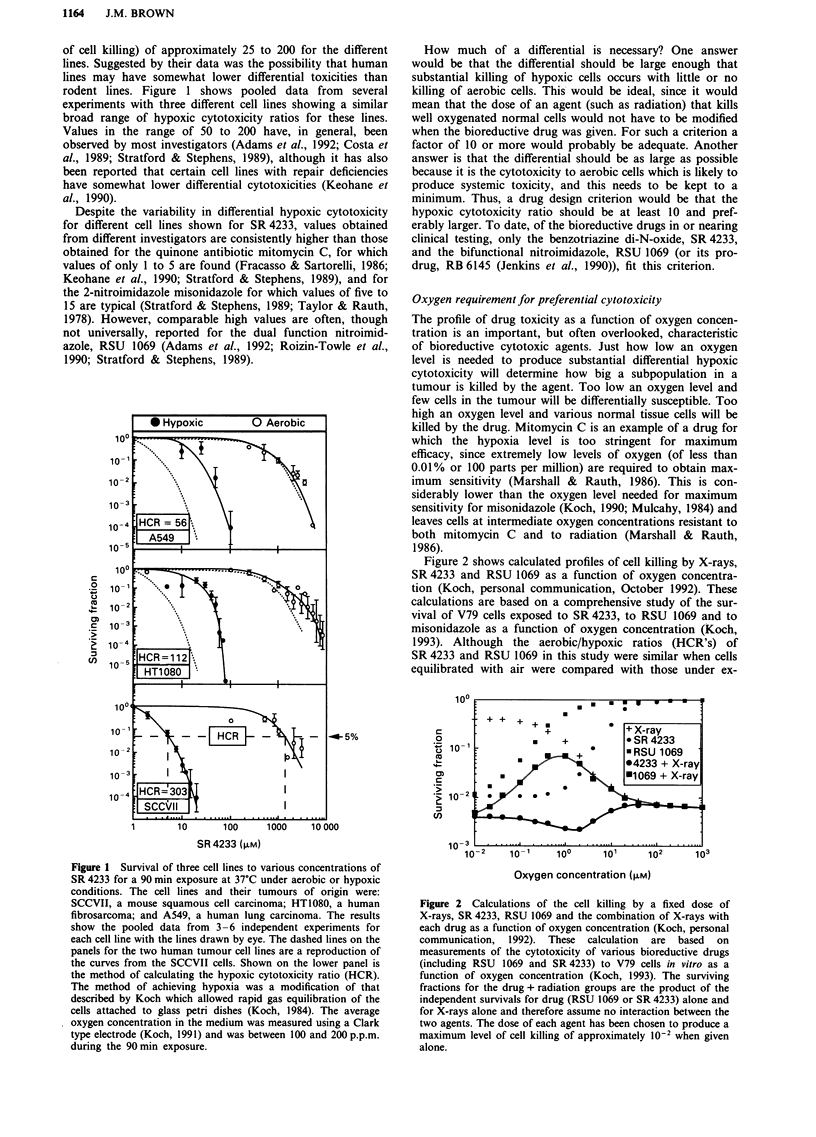
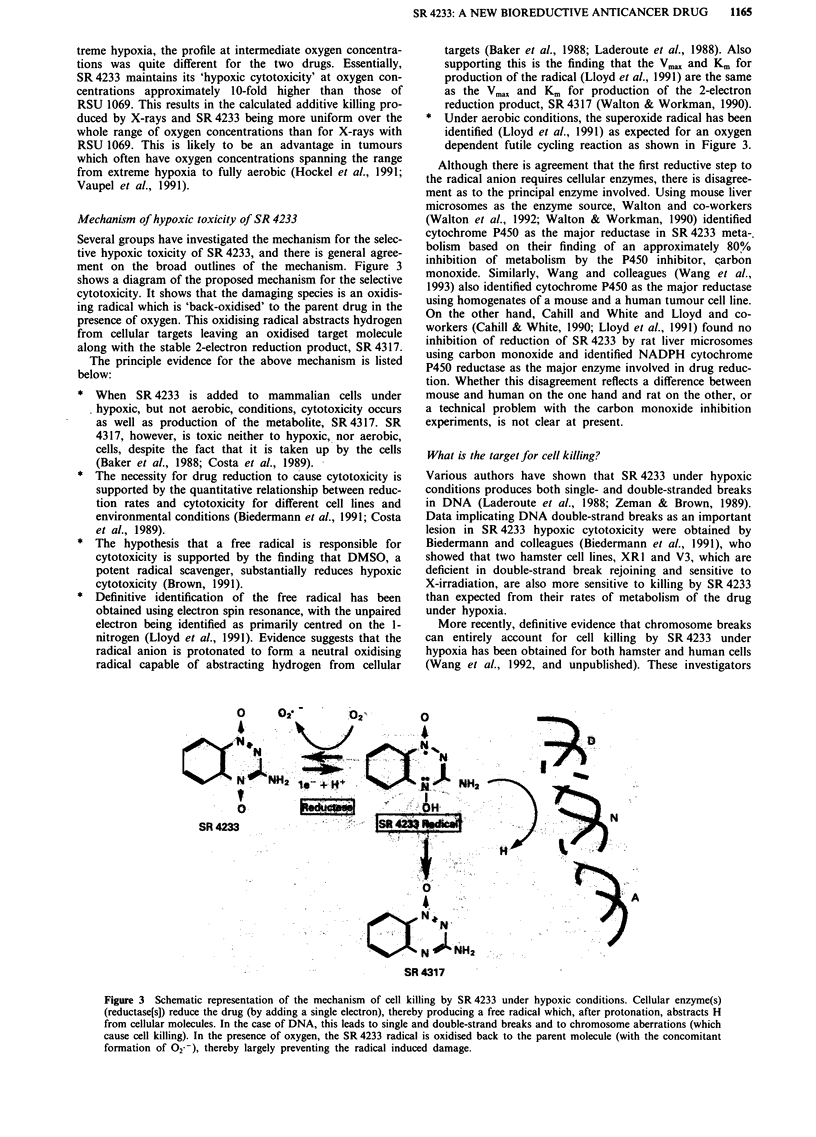
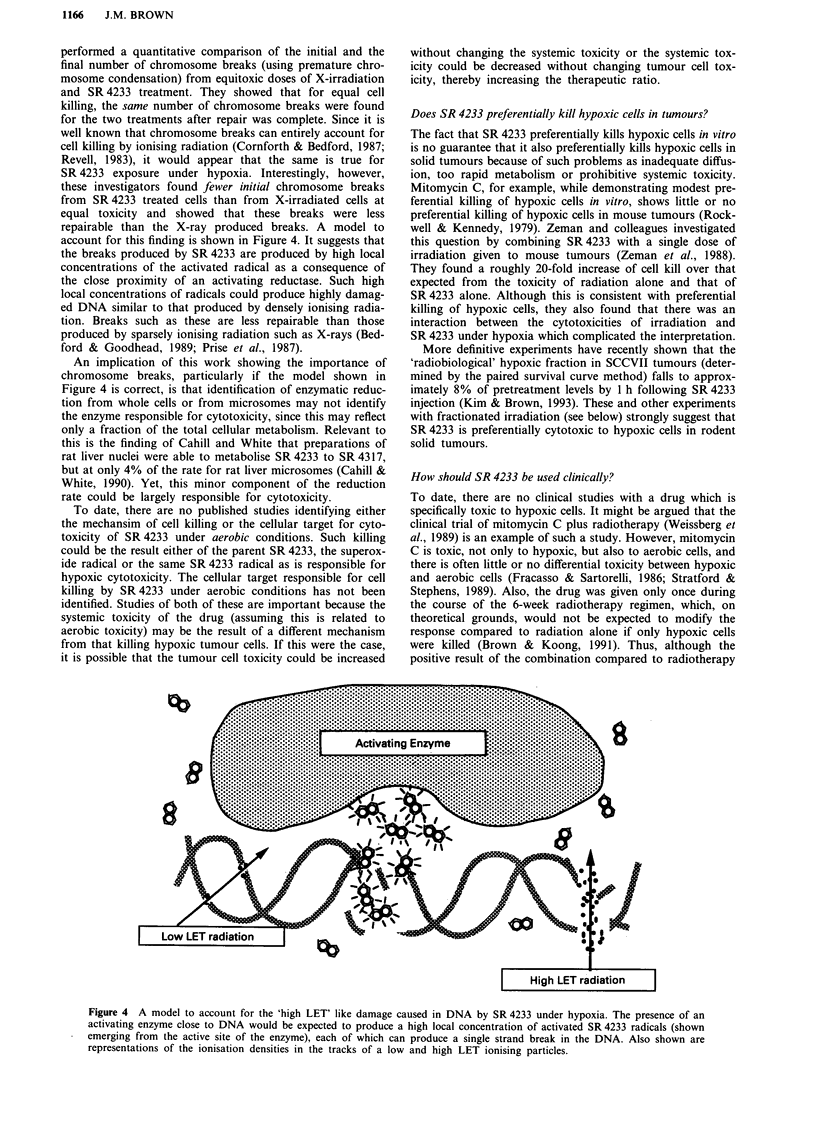
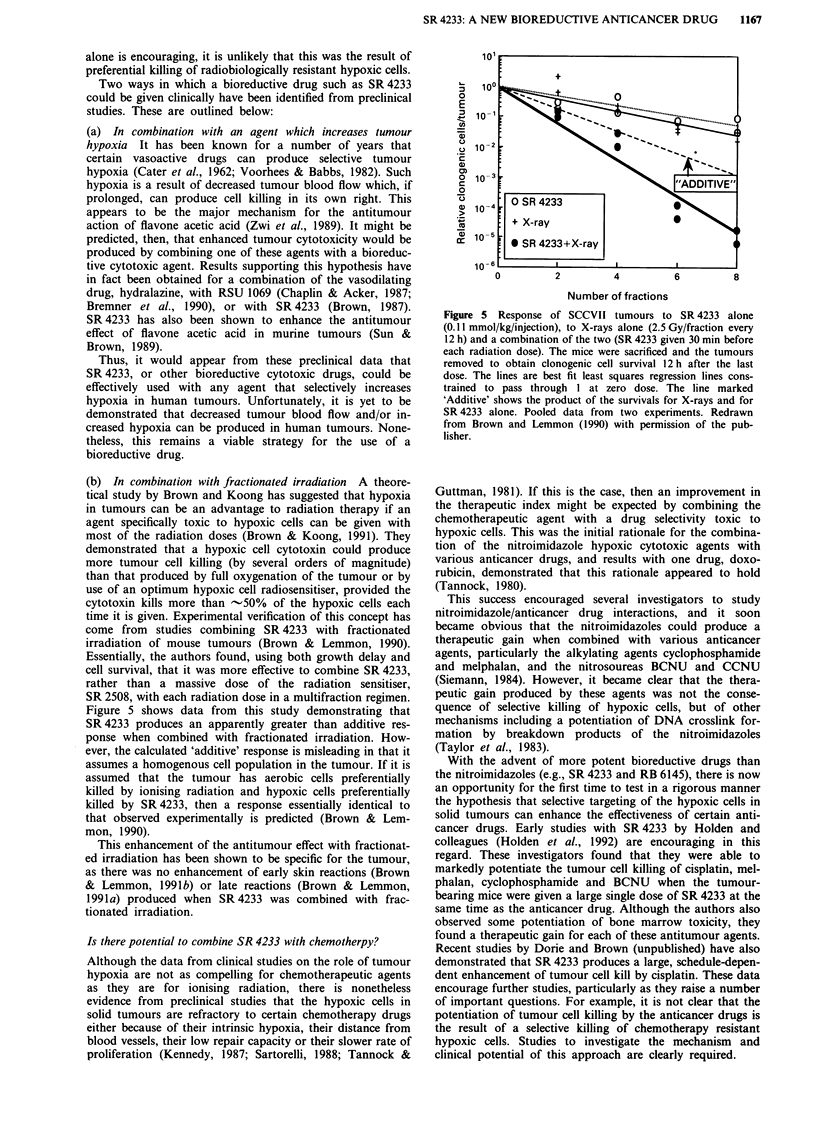
Abstract
SR 4233 (3-amino-1,2,4-benzotriazine 1,4-dioxide, WIN 59075, tirapazamine) is the lead compound in a new class of bioreductive anticancer drugs, the benzotriazine di-N-oxides. It is currently undergoing Phase I clinical testing. The preferential tumour cell killing of SR 4233 is a result of its high specific toxicity to cells at low oxygen tensions. Such hypoxic cells are a common feature of solid tumours, but not normal tissues, and are resistant to cancer therapies including radiation and some anticancer drugs. The killing of these tumour cells by SR 4233, particularly when given on multiple occasions, can increase total tumour cell killing by fractionated irradiation by several orders of magnitude without increasing toxicity to surrounding normal tissues. Topics covered in this review include the rationale for developing a hypoxic cytotoxic agent, the cytotoxicity of SR 4233 as a function of oxygen concentration, the mechanism of action of the drug and its intracellular target and the in vivo evidence that the drug may be useful as an adjunct both to radiotherapy and chemotherapy. Finally, the major unanswered questions on the drug are outlined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E. Hypoxia-mediated drugs for radiation and chemotherapy. Cancer. 1981 Aug 1;48(3):696–707. doi: 10.1002/1097-0142(19810801)48:3<696::aid-cncr2820480307>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Adams G. E., Stratford I. J., Edwards H. S., Bremner J. C., Cole S. Bioreductive drugs as post-irradiation sensitizers: comparison of dual function agents with SR 4233 and the mitomycin C analogue EO9. Int J Radiat Oncol Biol Phys. 1992;22(4):717–720. doi: 10.1016/0360-3016(92)90510-o. [DOI] [PubMed] [Google Scholar]
- Baker M. A., Zeman E. M., Hirst V. K., Brown J. M. Metabolism of SR 4233 by Chinese hamster ovary cells: basis of selective hypoxic cytotoxicity. Cancer Res. 1988 Nov 1;48(21):5947–5952. [PubMed] [Google Scholar]
- Bedford J. S., Goodhead D. T. Breakage of human interphase chromosomes by alpha particles and X-rays. Int J Radiat Biol. 1989 Feb;55(2):211–216. doi: 10.1080/09553008914550261. [DOI] [PubMed] [Google Scholar]
- Biedermann K. A., Wang J., Graham R. P., Brown J. M. SR 4233 cytotoxicity and metabolism in DNA repair-competent and repair-deficient cell cultures. Br J Cancer. 1991 Mar;63(3):358–362. doi: 10.1038/bjc.1991.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. C., Stratford I. J., Bowler J., Adams G. E. Bioreductive drugs and the selective induction of tumour hypoxia. Br J Cancer. 1990 May;61(5):717–721. doi: 10.1038/bjc.1990.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M. Keynote address: hypoxic cell radiosensitizers: where next? Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):987–993. doi: 10.1016/0360-3016(89)90901-2. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Koong A. Therapeutic advantage of hypoxic cells in tumors: a theoretical study. J Natl Cancer Inst. 1991 Feb 6;83(3):178–185. doi: 10.1093/jnci/83.3.178. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Lemmon M. J. Potentiation by the hypoxic cytotoxin SR 4233 of cell killing produced by fractionated irradiation of mouse tumors. Cancer Res. 1990 Dec 15;50(24):7745–7749. [PubMed] [Google Scholar]
- Brown J. M., Lemmon M. J. SR 4233: a tumor specific radiosensitizer active in fractionated radiation regimes. Radiother Oncol. 1991;20 (Suppl 1):151–156. doi: 10.1016/0167-8140(91)90203-s. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Lemmon M. J. Tumor hypoxia can be exploited to preferentially sensitize tumors to fractionated irradiation. Int J Radiat Oncol Biol Phys. 1991 Mar;20(3):457–461. doi: 10.1016/0360-3016(91)90057-b. [DOI] [PubMed] [Google Scholar]
- Bush R. S., Jenkin R. D., Allt W. E., Beale F. A., Bean H., Dembo A. J., Pringle J. F. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl. 1978 Jun;3:302–306. [PMC free article] [PubMed] [Google Scholar]
- CATER D. B., GRIGSON C. M., WATKINSON D. A. Changes of oxygen tension in tumours induced by vasoconstrictor and vasodilator drugs. Acta radiol. 1962 Dec;58:401–434. doi: 10.3109/00016926209169582. [DOI] [PubMed] [Google Scholar]
- Cahill A., White I. N. Reductive metabolism of 3-amino-1,2,4-benzotriazine-1,4-dioxide (SR 4233) and the induction of unscheduled DNA synthesis in rat and human derived cell lines. Carcinogenesis. 1990 Aug;11(8):1407–1411. doi: 10.1093/carcin/11.8.1407. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Acker B. The effect of hydralazine on the tumor cytotoxicity of the hypoxic cell cytotoxin RSU-1069: evidence for therapeutic gain. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):579–585. doi: 10.1016/0360-3016(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Horsman M. R., Aoki D. S. Nicotinamide, Fluosol DA and Carbogen: a strategy to reoxygenate acutely and chronically hypoxic cells in vivo. Br J Cancer. 1991 Jan;63(1):109–113. doi: 10.1038/bjc.1991.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornforth M. N., Bedford J. S. A quantitative comparison of potentially lethal damage repair and the rejoining of interphase chromosome breaks in low passage normal human fibroblasts. Radiat Res. 1987 Sep;111(3):385–405. [PubMed] [Google Scholar]
- Costa A. K., Baker M. A., Brown J. M., Trudell J. R. In vitro hepatotoxicity of SR 4233 (3-amino-1,2,4-benzotriazine-1,4-dioxide), a hypoxic cytotoxin and potential antitumor agent. Cancer Res. 1989 Feb 15;49(4):925–929. [PubMed] [Google Scholar]
- Fracasso P. M., Sartorelli A. C. Cytotoxicity and DNA lesions produced by mitomycin C and porfiromycin in hypoxic and aerobic EMT6 and Chinese hamster ovary cells. Cancer Res. 1986 Aug;46(8):3939–3944. [PubMed] [Google Scholar]
- Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988 May;14(5):831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Henk J. M., Smith C. W. Radiotherapy and hyperbaric oxygen in head and neck cancer. Interim report of second clinical trial. Lancet. 1977 Jul 16;2(8029):104–105. doi: 10.1016/s0140-6736(77)90117-9. [DOI] [PubMed] [Google Scholar]
- Holden S. A., Teicher B. A., Ara G., Herman T. S., Coleman C. N. Enhancement of alkylating agent activity by SR-4233 in the FSaIIC murine fibrosarcoma. J Natl Cancer Inst. 1992 Feb 5;84(3):187–193. doi: 10.1093/jnci/84.3.187. [DOI] [PubMed] [Google Scholar]
- Höckel M., Schlenger K., Knoop C., Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991 Nov 15;51(22):6098–6102. [PubMed] [Google Scholar]
- Jenkins T. C., Naylor M. A., O'Neill P., Threadgill M. D., Cole S., Stratford I. J., Adams G. E., Fielden E. M., Suto M. J., Stier M. A. Synthesis and evaluation of alpha-[[(2-haloethyl)amino]methyl]-2- nitro-1H-imidazole-1-ethanols as prodrugs of alpha-[(1-aziridinyl)methyl]-2- nitro-1H-imidazole-1-ethanol (RSU-1069) and its analogues which are radiosensitizers and bioreductively activated cytotoxins. J Med Chem. 1990 Sep;33(9):2603–2610. doi: 10.1021/jm00171a040. [DOI] [PubMed] [Google Scholar]
- Kennedy K. A. Hypoxic cells as specific drug targets for chemotherapy. Anticancer Drug Des. 1987 Oct;2(2):181–194. [PubMed] [Google Scholar]
- Keohane A., Godden J., Stratford I. J., Adams G. E. The effects of three bioreductive drugs (mitomycin C, RSU-1069 and SR4233) on cell lines selected for their sensitivity to mitomycin C or ionising radiation. Br J Cancer. 1990 May;61(5):722–726. doi: 10.1038/bjc.1990.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellen E., Joiner M. C., Collier J. M., Johns H., Rojas A. A therapeutic benefit from combining normobaric carbogen or oxygen with nicotinamide in fractionated X-ray treatments. Radiother Oncol. 1991 Oct;22(2):81–91. doi: 10.1016/0167-8140(91)90002-x. [DOI] [PubMed] [Google Scholar]
- Koch C. J. A thin-film culturing technique allowing rapid gas-liquid equilibration (6 sec) with no toxicity to mammalian cells. Radiat Res. 1984 Feb;97(2):434–442. [PubMed] [Google Scholar]
- Laderoute K., Wardman P., Rauth A. M. Molecular mechanisms for the hypoxia-dependent activation of 3-amino-1,2,4-benzotriazine-1,4-dioxide (SR 4233). Biochem Pharmacol. 1988 Apr 15;37(8):1487–1495. doi: 10.1016/0006-2952(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Lin A. J., Cosby L. A., Shansky C. W., Sartorelli A. C. Potential bioreductive alkylating agents. 1. Benzoquinone derivatives. J Med Chem. 1972 Dec;15(12):1247–1252. doi: 10.1021/jm00282a011. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Duling D. R., Rumyantseva G. V., Mason R. P., Bridson P. K. Microsomal reduction of 3-amino-1,2,4-benzotriazine 1,4-dioxide to a free radical. Mol Pharmacol. 1991 Sep;40(3):440–445. [PubMed] [Google Scholar]
- Marshall R. S., Rauth A. M. Modification of the cytotoxic activity of mitomycin C by oxygen and ascorbic acid in Chinese hamster ovary cells and a repair-deficient mutant. Cancer Res. 1986 Jun;46(6):2709–2713. [PubMed] [Google Scholar]
- Moore H. W. Bioactivation as a model for drug design bioreductive alkylation. Science. 1977 Aug 5;197(4303):527–532. doi: 10.1126/science.877572. [DOI] [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Tumor hypoxia: its impact on cancer therapy. Cancer Metastasis Rev. 1987;5(4):313–341. doi: 10.1007/BF00055376. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W., Vaupel P., Manz R., Schmidseder R. Intracapillary oxyhemoglobin saturation of malignant tumors in humans. Int J Radiat Oncol Biol Phys. 1981 Oct;7(10):1397–1404. doi: 10.1016/0360-3016(81)90036-5. [DOI] [PubMed] [Google Scholar]
- Mulcahy R. T. Effect of oxygen on misonidazole chemosensitization and cytotoxicity in vitro. Cancer Res. 1984 Oct;44(10):4409–4413. [PubMed] [Google Scholar]
- Overgaard J., Hansen H. S., Jørgensen K., Hjelm Hansen M. Primary radiotherapy of larynx and pharynx carcinoma--an analysis of some factors influencing local control and survival. Int J Radiat Oncol Biol Phys. 1986 Apr;12(4):515–521. doi: 10.1016/0360-3016(86)90058-1. [DOI] [PubMed] [Google Scholar]
- Prise K. M., Davies S., Michael B. D. The relationship between radiation-induced DNA double-strand breaks and cell kill in hamster V79 fibroblasts irradiated with 250 kVp X-rays, 2.3 MeV neutrons or 238Pu alpha-particles. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Dec;52(6):893–902. doi: 10.1080/09553008714552481. [DOI] [PubMed] [Google Scholar]
- Rockwell S., Kennedy K. A. Combination therapy with radiation and mitomycin C: preliminary results with EMT6 tumor cells in vitro and in vivo. Int J Radiat Oncol Biol Phys. 1979 Sep;5(9):1673–1676. doi: 10.1016/0360-3016(79)90795-8. [DOI] [PubMed] [Google Scholar]
- Rockwell S., Kennedy K. A., Sartorelli A. C. Mitomycin-C as a prototype bioreductive alkylating agent: in vitro studies of metabolism and cytotoxicity. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):753–755. doi: 10.1016/0360-3016(82)90728-3. [DOI] [PubMed] [Google Scholar]
- Rockwell S., Moulder J. E. Hypoxic fractions of human tumors xenografted into mice: a review. Int J Radiat Oncol Biol Phys. 1990 Jul;19(1):197–202. doi: 10.1016/0360-3016(90)90154-c. [DOI] [PubMed] [Google Scholar]
- Roizin-Towle L., Pirro J. P., Hall E. J. Studies with bifunctional bioreductive drugs. II. Cytotoxicity assayed with A-549 lung carcinoma cells of human origin. Radiat Res. 1990 Oct;124(1 Suppl):S50–S55. [PubMed] [Google Scholar]
- Sartorelli A. C. Therapeutic attack of hypoxic cells of solid tumors: presidential address. Cancer Res. 1988 Feb 15;48(4):775–778. [PubMed] [Google Scholar]
- Siemann D. W. Modification of chemotherapy by nitroimidazoles. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1585–1594. doi: 10.1016/0360-3016(84)90508-x. [DOI] [PubMed] [Google Scholar]
- Spiegel J. F., Spear M. A., Brown J. M. Toxicology of daily administration to mice of the radiation potentiator SR 4233 (WIN 59075). Radiother Oncol. 1993 Jan;26(1):79–81. doi: 10.1016/0167-8140(93)90031-3. [DOI] [PubMed] [Google Scholar]
- Stratford I. J., O'Neill P., Sheldon P. W., Silver A. R., Walling J. M., Adams G. E. RSU 1069, a nitroimidazole containing an aziridine group. Bioreduction greatly increases cytotoxicity under hypoxic conditions. Biochem Pharmacol. 1986 Jan 1;35(1):105–109. doi: 10.1016/0006-2952(86)90566-6. [DOI] [PubMed] [Google Scholar]
- Stratford I. J., Stephens M. A. The differential hypoxic cytotoxicity of bioreductive agents determined in vitro by the MTT assay. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):973–976. doi: 10.1016/0360-3016(89)90898-5. [DOI] [PubMed] [Google Scholar]
- Sun J. R., Brown J. M. Enhancement of the antitumor effect of flavone acetic acid by the bioreductive cytotoxic drug SR 4233 in a murine carcinoma. Cancer Res. 1989 Oct 15;49(20):5664–5670. [PubMed] [Google Scholar]
- Tannock I. F. In vivo interaction of anti-cancer drugs with misonidazole or metronidazole: methotrexate, 5-fluorouracil and adriamycin. Br J Cancer. 1980 Dec;42(6):861–870. doi: 10.1038/bjc.1980.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I., Guttman P. Response of Chinese hamster ovary cells to anticancer drugs under aerobic and hypoxic conditions. Br J Cancer. 1981 Feb;43(2):245–248. doi: 10.1038/bjc.1981.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor Y. C., Evans J. W., Brown J. M. Mechanism of sensitization of Chinese hamster ovary cells to melphalan by hypoxic treatment with misonidazole. Cancer Res. 1983 Jul;43(7):3175–3181. [PubMed] [Google Scholar]
- Taylor Y. C., Rauth A. M. Differences in the toxicity and metabolism of the 2-nitroimidazole misonidazole (Ro-07-0582) in HeLa and Chinese hamster ovary cells. Cancer Res. 1978 Sep;38(9):2745–2752. [PubMed] [Google Scholar]
- Vaupel P., Schlenger K., Knoop C., Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991 Jun 15;51(12):3316–3322. [PubMed] [Google Scholar]
- Voorhees W. D., 3rd, Babbs C. F. Hydralazine-enhanced selective heating of transmissible venereal tumor implants in dogs. Eur J Cancer Clin Oncol. 1982 Oct;18(10):1027–1033. doi: 10.1016/0277-5379(82)90252-8. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Wolf C. R., Workman P. The role of cytochrome P450 and cytochrome P450 reductase in the reductive bioactivation of the novel benzotriazine di-N-oxide hypoxic cytotoxin 3-amino-1,2,4-benzotriazine-1,4-dioxide (SR 4233, WIN 59075) by mouse liver. Biochem Pharmacol. 1992 Jul 22;44(2):251–259. doi: 10.1016/0006-2952(92)90007-6. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Workman P. Enzymology of the reductive bioactivation of SR 4233. A novel benzotriazine di-N-oxide hypoxic cell cytotoxin. Biochem Pharmacol. 1990 Jun 1;39(11):1735–1742. doi: 10.1016/0006-2952(90)90119-6. [DOI] [PubMed] [Google Scholar]
- Wang J., Biedermann K. A., Brown J. M. Repair of DNA and chromosome breaks in cells exposed to SR 4233 under hypoxia or to ionizing radiation. Cancer Res. 1992 Aug 15;52(16):4473–4477. [PubMed] [Google Scholar]
- Wang J., Biedermann K. A., Wolf C. R., Brown J. M. Metabolism of the bioreductive cytotoxin SR 4233 by tumour cells: enzymatic studies. Br J Cancer. 1993 Feb;67(2):321–325. doi: 10.1038/bjc.1993.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg J. B., Son Y. H., Papac R. J., Sasaki C., Fischer D. B., Lawrence R., Rockwell S., Sartorelli A. C., Fischer J. J. Randomized clinical trial of mitomycin C as an adjunct to radiotherapy in head and neck cancer. Int J Radiat Oncol Biol Phys. 1989 Jul;17(1):3–9. doi: 10.1016/0360-3016(89)90362-3. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M., Lemmon M. J., Hirst V. K., Lee W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M. Pre- and post-irradiation radiosensitization by SR 4233. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):967–971. doi: 10.1016/0360-3016(89)90897-3. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Hirst V. K., Lemmon M. J., Brown J. M. Enhancement of radiation-induced tumor cell killing by the hypoxic cell toxin SR 4233. Radiother Oncol. 1988 Jul;12(3):209–218. doi: 10.1016/0167-8140(88)90263-0. [DOI] [PubMed] [Google Scholar]
- Zwi L. J., Baguley B. C., Gavin J. B., Wilson W. R. Blood flow failure as a major determinant in the antitumor action of flavone acetic acid. J Natl Cancer Inst. 1989 Jul 5;81(13):1005–1013. doi: 10.1093/jnci/81.13.1005. [DOI] [PubMed] [Google Scholar]



