Abstract
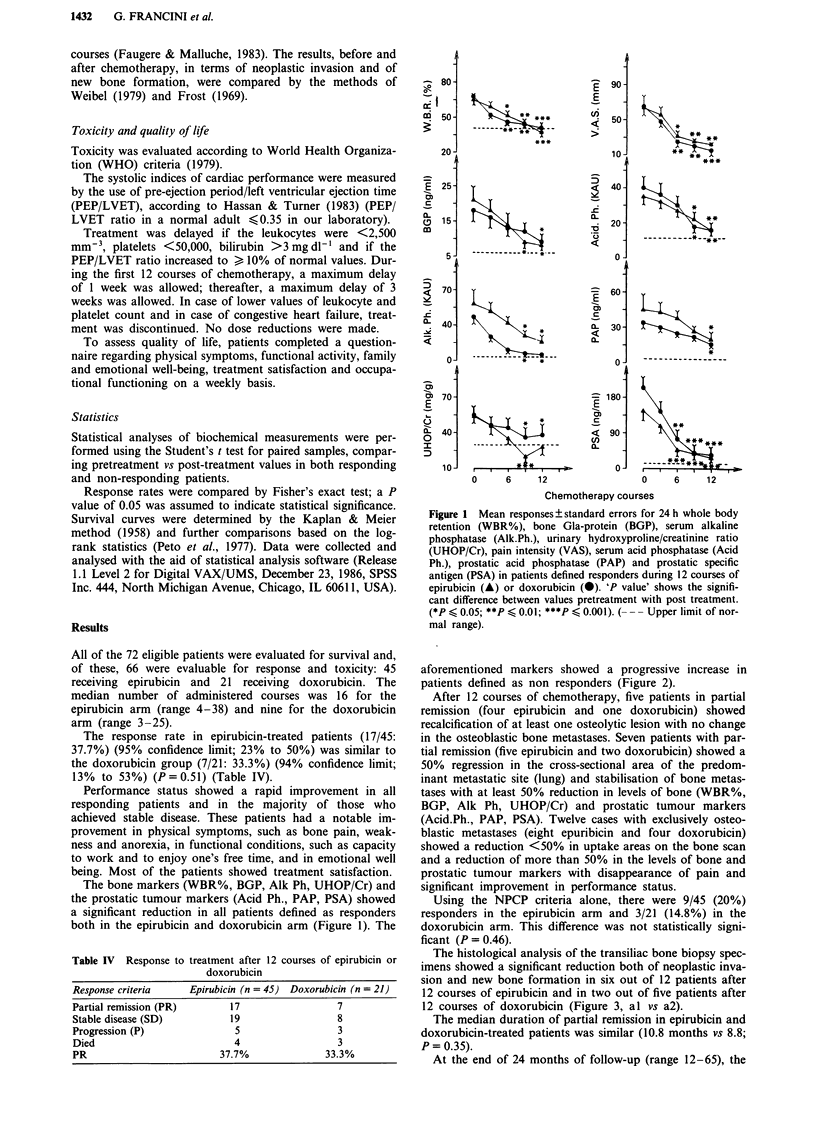
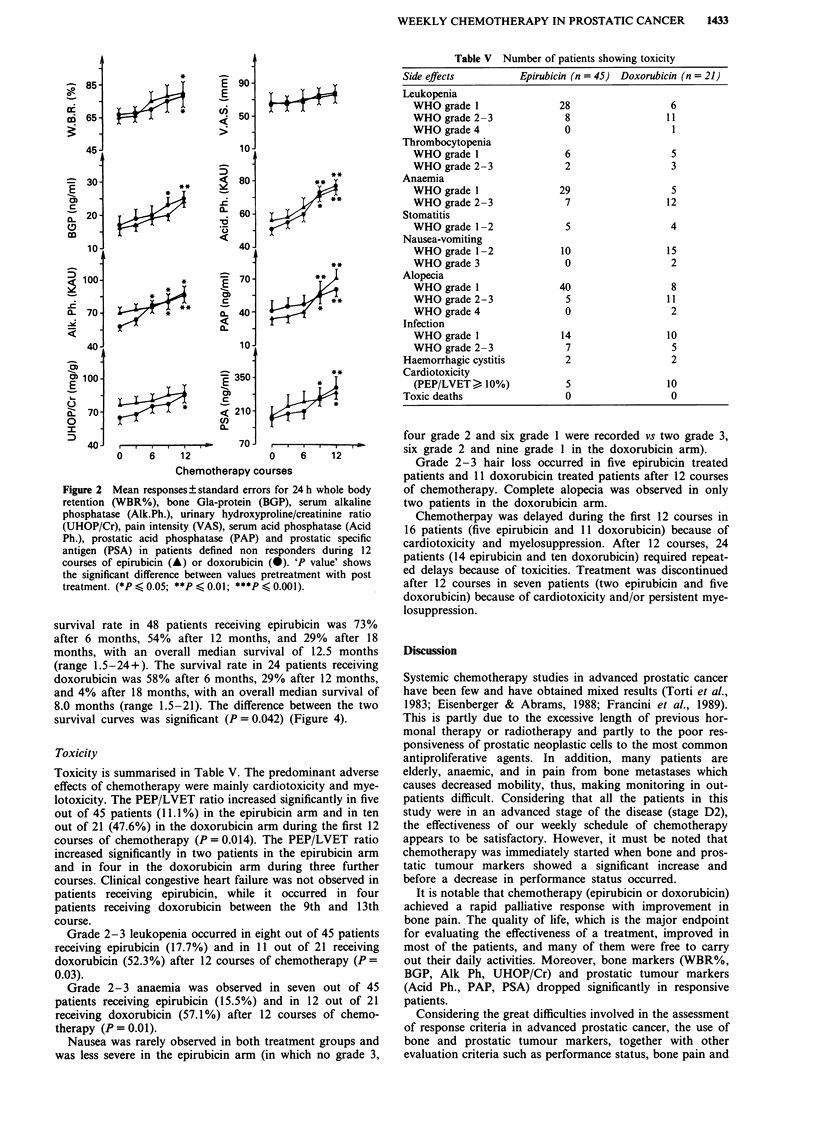
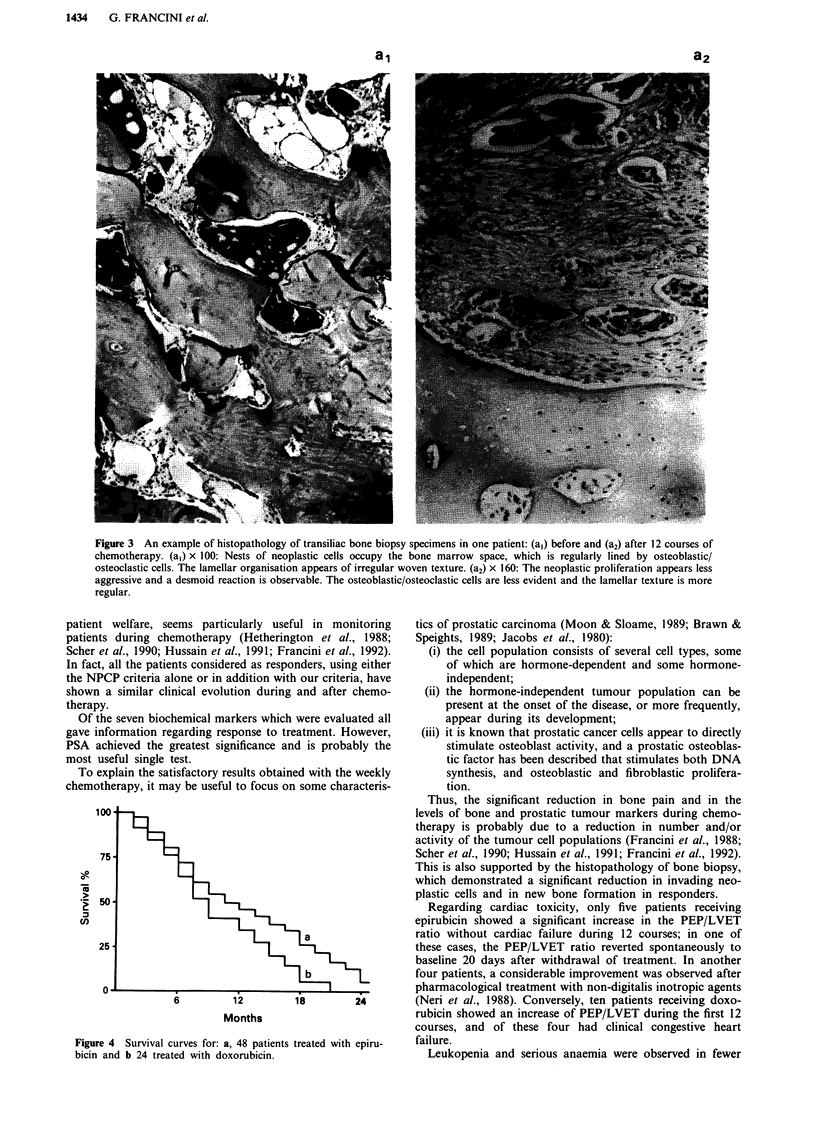
This randomised phase II study was performed in order to evaluate the effectiveness of a weekly chemotherapy regimen in advanced prostatic carcinoma patients (stage D2) refractory to hormonal therapy. Seventy-two cases were studied: they were randomised in a 2:1 ratio to receive either epirubicin (30 mg m-2 weekly) or doxorubicin (25 mg m-2 weekly); 48 patients received epirubicin and 24 received doxorubicin. After 12 courses of chemotherapy, the 45 evaluable patients in the epirubicin arm showed a response rate of 37.7% and the 21 evaluable patients in the doxorubicin arm showed a response rate of 33.3% (P = 0.51). Pain intensity, bone and prostatic tumour markers rapidly and significantly decreased in responders. An improvement in physical symptoms, functional conditions and in emotional well-being was observed in the majority of the treated patients. The histological analysis of bone metastases, performed before and after 12 courses of chemotherapy showed a significant reduction in neoplastic invasion and in new bone formation in responders. Cardiac performance worsened in five out of 45 patients and in ten out of 21 during the first 12 courses of epirubicin or doxorubicin respectively (P = 0.014). The median survival was 12.5 months in the epirubicin arm and 8.0 months in the doxorubicin arm (P = 0.042). Our data indicate that in advanced prostatic carcinoma, a weekly epirubicin regimen may give rapid palliative results, similar to that of doxorubicin, but with less side-effects.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmann F. R., Schifman R. B. Prospective comparison between serum monoclonal prostate specific antigen and acid phosphatase measurements in metastatic prostatic cancer. J Urol. 1987 Mar;137(3):431–434. doi: 10.1016/s0022-5347(17)44057-2. [DOI] [PubMed] [Google Scholar]
- Berry W. R., Laszlo J., Cox E., Walker A., Paulson D. Prognostic factors in metastatic and hormonally unresponsive carcinoma of the prostate. Cancer. 1979 Aug;44(2):763–775. doi: 10.1002/1097-0142(197908)44:2<763::aid-cncr2820440251>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Brawn P. N., Speights V. O. The dedifferentiation of metastatic prostate carcinoma. Br J Cancer. 1989 Jan;59(1):85–88. doi: 10.1038/bjc.1989.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniggia A., Vattimo A. Kinetics of 99mtechnetium-tin-methylene-diphosphonate in normal subjects and pathological conditions: a simple index of bone metabolism. Calcif Tissue Int. 1980;30(1):5–13. doi: 10.1007/BF02408600. [DOI] [PubMed] [Google Scholar]
- DeWys W. D., Bauer M., Colsky J., Cooper R. A., Creech R., Carbone P. P. Comparative trial of adriamycin and 5-fluorouracil in advanced prostatic cancer--progress report. Cancer Treat Rep. 1977 Mar-Apr;61(2):325–328. [PubMed] [Google Scholar]
- Eisenberger M. A., Abrams J. S. Chemotherapy for prostatic carcinoma. Semin Urol. 1988 Nov;6(4):303–310. [PubMed] [Google Scholar]
- Faugere M. C., Malluche H. H. Comparison of different bone-biopsy techniques for qualitative and quantitative diagnosis of metabolic bone diseases. J Bone Joint Surg Am. 1983 Dec;65(9):1314–1318. [PubMed] [Google Scholar]
- Fosså S. D., Hosbach G., Paus E. Flutamide in hormone-resistant prostatic cancer. J Urol. 1990 Dec;144(6):1411–1414. doi: 10.1016/s0022-5347(17)39756-2. [DOI] [PubMed] [Google Scholar]
- Frost H. M. Tetracycline-based histological analysis of bone remodeling. Calcif Tissue Res. 1969;3(3):211–237. doi: 10.1007/BF02058664. [DOI] [PubMed] [Google Scholar]
- Hassan S., Turner P. Systolic time intervals: a review of the method in the non-invasive investigation of cardiac function in health, disease and clinical pharmacology. Postgrad Med J. 1983 Jul;59(693):423–434. doi: 10.1136/pgmj.59.693.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington J. W., Siddall J. K., Cooper E. H. Contribution of bone scintigraphy, prostatic acid phosphatase and prostate-specific antigen to the monitoring of prostatic cancer. Eur Urol. 1988;14(1):1–5. doi: 10.1159/000472885. [DOI] [PubMed] [Google Scholar]
- Immergut M., Nordschow C. D., Tammes A. R., Flocks R. H. Urinary hydroxyproline excretion in patients with metastatic carcinoma of the prostate. J Urol. 1966 Oct;96(4):570–572. doi: 10.1016/S0022-5347(17)63309-3. [DOI] [PubMed] [Google Scholar]
- Jacobs S. C., Pikna D., Lawson R. K. Prostatic osteoblastic factor. Invest Urol. 1979 Nov;17(3):195–198. [PubMed] [Google Scholar]
- Kelsen D., Atiq O. T., Saltz L., Niedzwiecki D., Ginn D., Chapman D., Heelan R., Lightdale C., Vinciguerra V., Brennan M. FAMTX versus etoposide, doxorubicin, and cisplatin: a random assignment trial in gastric cancer. J Clin Oncol. 1992 Apr;10(4):541–548. doi: 10.1200/JCO.1992.10.4.541. [DOI] [PubMed] [Google Scholar]
- Klein L. A. Prostatic carcinoma. N Engl J Med. 1979 Apr 12;300(15):824–833. doi: 10.1056/NEJM197904123001504. [DOI] [PubMed] [Google Scholar]
- Levenson R. M., Sauerbrunn B. J., Bates H. R., Newman R. D., Eddy J. L., Ihde D. C. Comparative value of bone scintigraphy and radiography in monitoring tumor response in systemically treated prostatic carcinoma. Radiology. 1983 Feb;146(2):513–518. doi: 10.1148/radiology.146.2.6294738. [DOI] [PubMed] [Google Scholar]
- Moon T. D., Sloane B. B. Prostatic adenocarcinoma. Carcinogenesis and growth. J Am Geriatr Soc. 1989 Jan;37(1):55–64. doi: 10.1111/j.1532-5415.1989.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Neri B., Neri G. C., Bandinelli M. Differences between carnitine derivatives and coenzyme Q10 in preventing in vitro doxorubicin-related cardiac damages. Oncology. 1988;45(3):242–246. doi: 10.1159/000226569. [DOI] [PubMed] [Google Scholar]
- Newling D. W. Criteria for response to treatment of metastatic prostatic cancer. Prog Clin Biol Res. 1985;185A:205–220. [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Williamson M. K., Lothringer J. W. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem. 1981 Dec 25;256(24):12760–12766. [PubMed] [Google Scholar]
- Reif A. E., Schlesinger R. M., Fish C. A., Robinson C. M. Acid phosphatase isozymes in cancer of the prostate. Cancer. 1973 Mar;31(3):689–699. doi: 10.1002/1097-0142(197303)31:3<689::aid-cncr2820310331>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Scher H. I., Curley T., Geller N., Engstrom C., Dershaw D. D., Lin S. Y., Fitzpatrick K., Nisselbaum J., Schwartz M., Bezirdjian L. Trimetrexate in prostatic cancer: preliminary observations on the use of prostate-specific antigen and acid phosphatase as a marker in measurable hormone-refractory disease. J Clin Oncol. 1990 Nov;8(11):1830–1838. doi: 10.1200/JCO.1990.8.11.1830. [DOI] [PubMed] [Google Scholar]
- Scott J., Huskisson E. C. Graphic representation of pain. Pain. 1976 Jun;2(2):175–184. [PubMed] [Google Scholar]
- Sharifi R., Soloway M. Clinical study of leuprolide depot formulation in the treatment of advanced prostate cancer. The Leuprolide Study Group. J Urol. 1990 Jan;143(1):68–71. doi: 10.1016/s0022-5347(17)39868-3. [DOI] [PubMed] [Google Scholar]
- Slack N. H., Murphy G. P. Criteria for evaluating patient responses to treatment modalities for prostatic cancer. Urol Clin North Am. 1984 May;11(2):337–342. [PubMed] [Google Scholar]
- Torti F. M., Aston D., Lum B. L., Kohler M., Williams R., Spaulding J. T., Shortliffe L., Freiha F. S. Weekly doxorubicin in endocrine-refractory carcinoma of the prostate. J Clin Oncol. 1983 Aug;1(8):477–482. doi: 10.1200/JCO.1983.1.8.477. [DOI] [PubMed] [Google Scholar]
- Torti F. M., Bristow M. M., Lum B. L., Carter S. K., Howes A. E., Aston D. A., Brown B. W., Jr, Hannigan J. F., Jr, Meyers F. J., Mitchell E. P. Cardiotoxicity of epirubicin and doxorubicin: assessment by endomyocardial biopsy. Cancer Res. 1986 Jul;46(7):3722–3727. [PubMed] [Google Scholar]
- Von Hoff D. D., Rozencweig M., Piccart M. The cardiotoxicity of anticancer agents. Semin Oncol. 1982 Mar;9(1):23–33. [PubMed] [Google Scholar]
- Wajsman Z., Chu T. M., Bross D., Saroff J., Murphy G. P., Johnson D. E., Scott W. W., Gibbons R. P., Prout G. R., Schmidt J. D. Clinical significance of serum alkaline phosphatase isoenzyme levels in advanced prostatic carcinoma. J Urol. 1978 Feb;119(2):244–246. doi: 10.1016/s0022-5347(17)57446-7. [DOI] [PubMed] [Google Scholar]




