Abstract
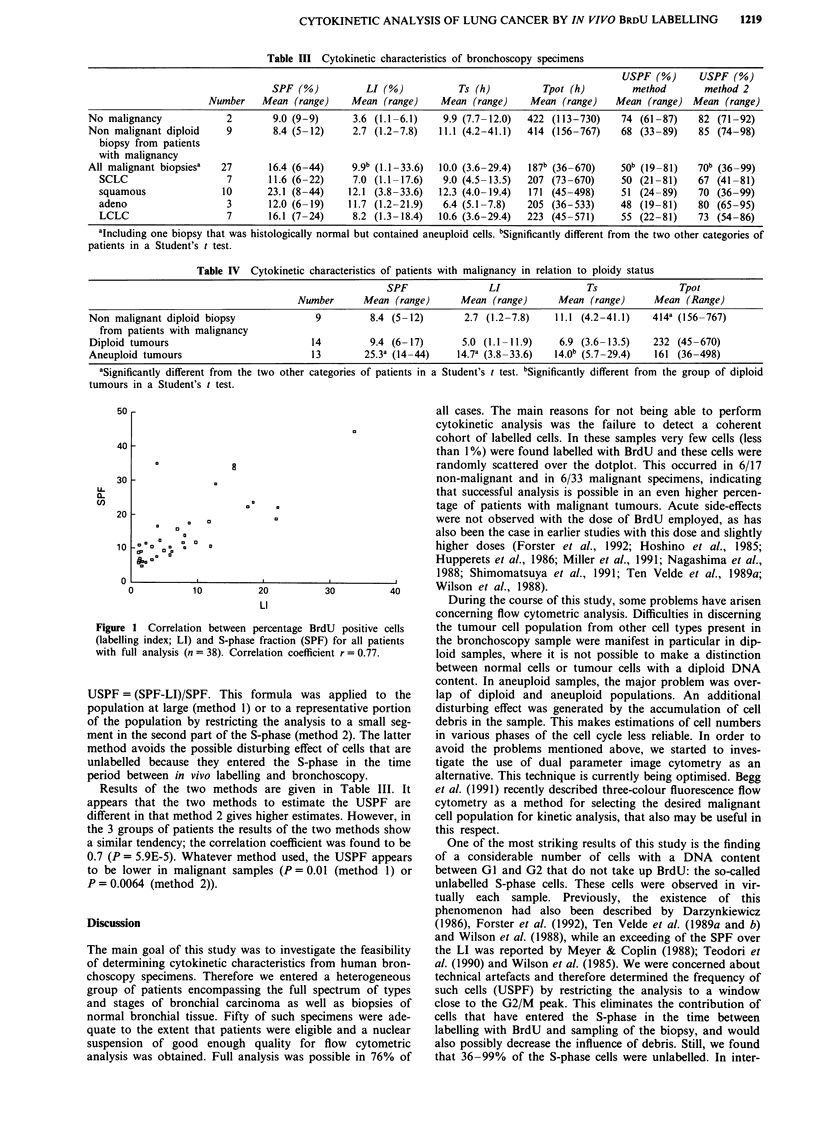
Cytokinetic parameters of various types of lung cancer were determined in bronchoscopy specimens after in vivo labelling with the thymidine analogue bromodeoxyuridine (BrdU). The S-phase fraction and BrdU labelling index were measured flow cytometrically, allowing calculation of the S-phase transit time and potential tumour doubling time. The methodology used was found to be feasible for obtaining cytokinetic data from 76% of the bronchial biopsy samples. Despite the difference in clinical behaviour and growth pattern between small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), no significant differences were observed between the mean values of the cytokinetic parameters of SCLC and NSCLC. The estimated cell loss factor was higher in NSCLC than in SCLC. It appears that the growth of a tumour, as clinically observed, is to a considerable extent influenced by cell loss. In accord with this assumption is the fact that we have observed non-BrdU labelled S-phase cells, both in tumour biopsies and in apparently normal tissue. The presence of these so-called unlabelled S-phase cells in relation to cell loss is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baisch H., Beck H. P., Christensen I. J., Hartmann N. R., Fried J., Dean P. N., Gray J. W., Jett J. H., Johnston D. A., White R. A. A comparison of mathematical methods for the analysis of DNA histograms obtained by flow cytometry. Cell Tissue Kinet. 1982 May;15(3):235–249. doi: 10.1111/j.1365-2184.1982.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Begg A. C., Hofland I. Cell kinetic analysis of mixed populations using three-color fluorescence flow cytometry. Cytometry. 1991;12(5):445–454. doi: 10.1002/cyto.990120510. [DOI] [PubMed] [Google Scholar]
- Begg A. C., McNally N. J., Shrieve D. C., Kärcher H. A method to measure the duration of DNA synthesis and the potential doubling time from a single sample. Cytometry. 1985 Nov;6(6):620–626. doi: 10.1002/cyto.990060618. [DOI] [PubMed] [Google Scholar]
- Carlton J. C., Terry N. H., White R. A. Measuring potential doubling times of murine tumors using flow cytometry. Cytometry. 1991;12(7):645–650. doi: 10.1002/cyto.990120709. [DOI] [PubMed] [Google Scholar]
- Forster G., Cooke T. G., Cooke L. D., Stanton P. D., Bowie G., Stell P. M. Tumour growth rates in squamous carcinoma of the head and neck measured by in vivo bromodeoxyuridine incorporation and flow cytometry. Br J Cancer. 1992 May;65(5):698–702. doi: 10.1038/bjc.1992.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraire A. E., Johnson E. H., Yesner R., Zhang X. B., Spjut H. J., Greenberg S. D. Prognostic significance of histopathologic subtype and stage in small cell lung cancer. Hum Pathol. 1992 May;23(5):520–528. doi: 10.1016/0046-8177(92)90129-q. [DOI] [PubMed] [Google Scholar]
- Gatter K. C., Dunnill M. S., Gerdes J., Stein H., Mason D. Y. New approach to assessing lung tumours in man. J Clin Pathol. 1986 Jun;39(6):590–593. doi: 10.1136/jcp.39.6.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Nagashima T., Murovic J., Levin E. M., Levin V. A., Rupp S. M. Cell kinetic studies of in situ human brain tumors with bromodeoxyuridine. Cytometry. 1985 Nov;6(6):627–632. doi: 10.1002/cyto.990060619. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Hietanen T., Mattila J., Lehtinen M., Lauslahti K., Koivula T. Aneuploid DNA content and high S-phase fraction of tumour cells are related to poor prognosis in patients with primary breast cancer. Eur J Cancer Clin Oncol. 1987 Mar;23(3):277–282. doi: 10.1016/0277-5379(87)90071-x. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Coplin M. D. Thymidine labeling index, flow cytometric S-phase measurement, and DNA index in human tumors. Comparisons and correlations. Am J Clin Pathol. 1988 May;89(5):586–595. doi: 10.1093/ajcp/89.5.586. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Mazewski C. M., Yousuf N., Sheikh Y., White L. M., Yanik G. A., Hyams D. M., Lampkin B. C., Raza A. Simultaneous immunohistochemical detection of IUdR and BrdU infused intravenously to cancer patients. J Histochem Cytochem. 1991 Apr;39(4):407–412. doi: 10.1177/39.4.2005370. [DOI] [PubMed] [Google Scholar]
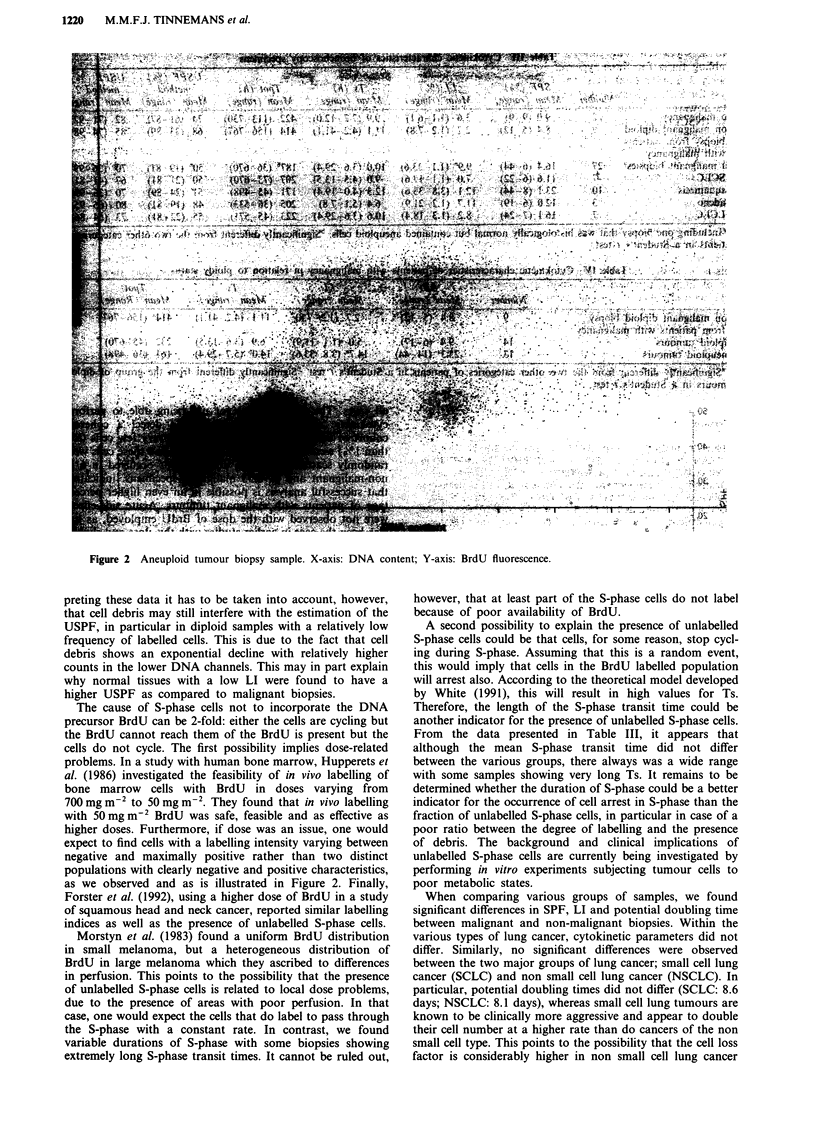
- Morstyn G., Hsu S. M., Kinsella T., Gratzner H., Russo A., Mitchell J. B. Bromodeoxyuridine in tumors and chromosomes detected with a monoclonal antibody. J Clin Invest. 1983 Nov;72(5):1844–1850. doi: 10.1172/JCI111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima T., Hoshino T., Cho K. G., Edwards M. S., Hudgins R. J., Davis R. L. The proliferative potential of human ependymomas measured by in situ bromodeoxyuridine labeling. Cancer. 1988 Jun 15;61(12):2433–2438. doi: 10.1002/1097-0142(19880615)61:12<2433::aid-cncr2820611207>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- O'Reilly S. M., Camplejohn R. S., Barnes D. M., Millis R. R., Allen D., Rubens R. D., Richards M. A. DNA index, S-phase fraction, histological grade and prognosis in breast cancer. Br J Cancer. 1990 May;61(5):671–674. doi: 10.1038/bjc.1990.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte B., Reynders M. M., van Assche C. L., Hupperets P. S., Bosman F. T., Blijham G. H. An improved method for the immunocytochemical detection of bromodeoxyuridine labeled nuclei using flow cytometry. Cytometry. 1987 Jul;8(4):372–376. doi: 10.1002/cyto.990080405. [DOI] [PubMed] [Google Scholar]
- Shimomatsuya T., Tanigawa N., Muraoka R. Proliferative activity of human tumors: assessment using bromodeoxyuridine and flow cytometry. Jpn J Cancer Res. 1991 Mar;82(3):357–362. doi: 10.1111/j.1349-7006.1991.tb01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomro I. N., Whimster W. F. Growth fraction in lung tumours determined by Ki67 immunostaining and comparison with AgNOR scores. J Pathol. 1990 Nov;162(3):217–222. doi: 10.1002/path.1711620307. [DOI] [PubMed] [Google Scholar]
- Teodori L., Trinca M. L., Goehde W., Hemmer J., Salvati F., Storniello G., Mauro F. Cytokinetic investigation of lung tumors using the anti-bromodeoxyuridine (BUdR) monoclonal antibody method: comparison with DNA flow cytometric data. Int J Cancer. 1990 Jun 15;45(6):995–1001. doi: 10.1002/ijc.2910450602. [DOI] [PubMed] [Google Scholar]
- Terz J. J., Curutchet H. P., Lawrence W., Jr Analysis of the cell kinetics of human solid tumors. Cancer. 1971 Nov;28(5):1100–1110. doi: 10.1002/1097-0142(1971)28:5<1100::aid-cncr2820280503>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Tubiana M., Courdi A. Cell proliferation kinetics in human solid tumors: relation to probability of metastatic dissemination and long-term survival. Radiother Oncol. 1989 May;15(1):1–18. doi: 10.1016/0167-8140(89)90113-8. [DOI] [PubMed] [Google Scholar]
- Volm M., Mattern J., Sonka J., Vogt-Schaden M., Wayss K. DNA distribution in non-small-cell lung carcinomas and its relationship to clinical behavior. Cytometry. 1985 Jul;6(4):348–356. doi: 10.1002/cyto.990060412. [DOI] [PubMed] [Google Scholar]
- White R. A. A theory for analysis of cell populations with non-cycling S phase cells. J Theor Biol. 1991 May 21;150(2):201–214. doi: 10.1016/s0022-5193(05)80332-7. [DOI] [PubMed] [Google Scholar]
- White R. A., Terry N. H., Meistrich M. L., Calkins D. P. Improved method for computing potential doubling time from flow cytometric data. Cytometry. 1990;11(2):314–317. doi: 10.1002/cyto.990110214. [DOI] [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dische S., Saunders M. I., Des Rochers C., Lewis A. A., Bennett M. H. Measurement of cell kinetics in human tumours in vivo using bromodeoxyuridine incorporation and flow cytometry. Br J Cancer. 1988 Oct;58(4):423–431. doi: 10.1038/bjc.1988.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dunphy E., Kärcher H., Pfragner R. The labelling index of human and mouse tumours assessed by bromodeoxyuridine staining in vitro and in vivo and flow cytometry. Cytometry. 1985 Nov;6(6):641–647. doi: 10.1002/cyto.990060621. [DOI] [PubMed] [Google Scholar]
- ten Velde G. P., Schutte B., Reijnders M. M., Bosman F. T., Blijham G. H. Cytokinetic analysis of lung cancer by bromodeoxyuridine labeling of cytology specimens. Cytometry. 1989 Nov;10(6):807–810. doi: 10.1002/cyto.990100621. [DOI] [PubMed] [Google Scholar]
- ten Velde G. P., Schutte B., Vermeulen A., Volovics A., Reynders M. M., Blijham G. H. Flow cytometric analysis of DNA ploidy level in paraffin-embedded tissue of non-small-cell lung cancer. Eur J Cancer Clin Oncol. 1988 Mar;24(3):455–460. doi: 10.1016/s0277-5379(98)90016-5. [DOI] [PubMed] [Google Scholar]


