Abstract
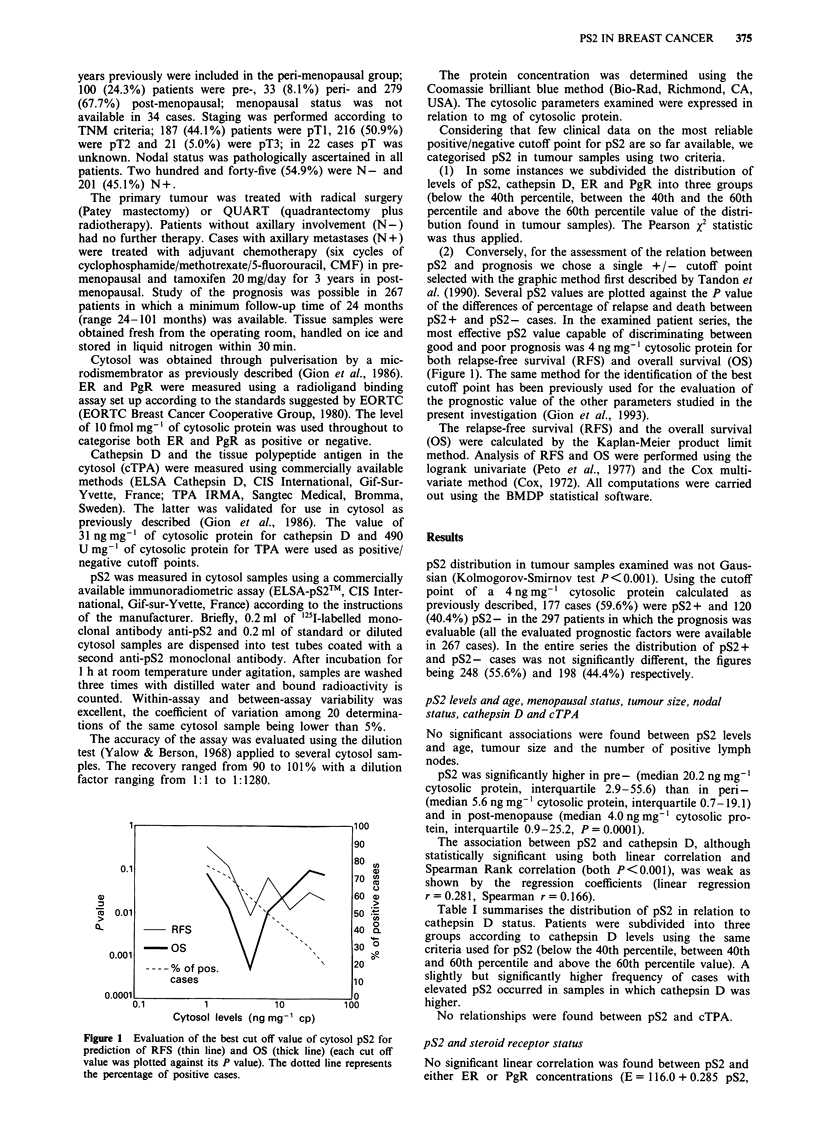
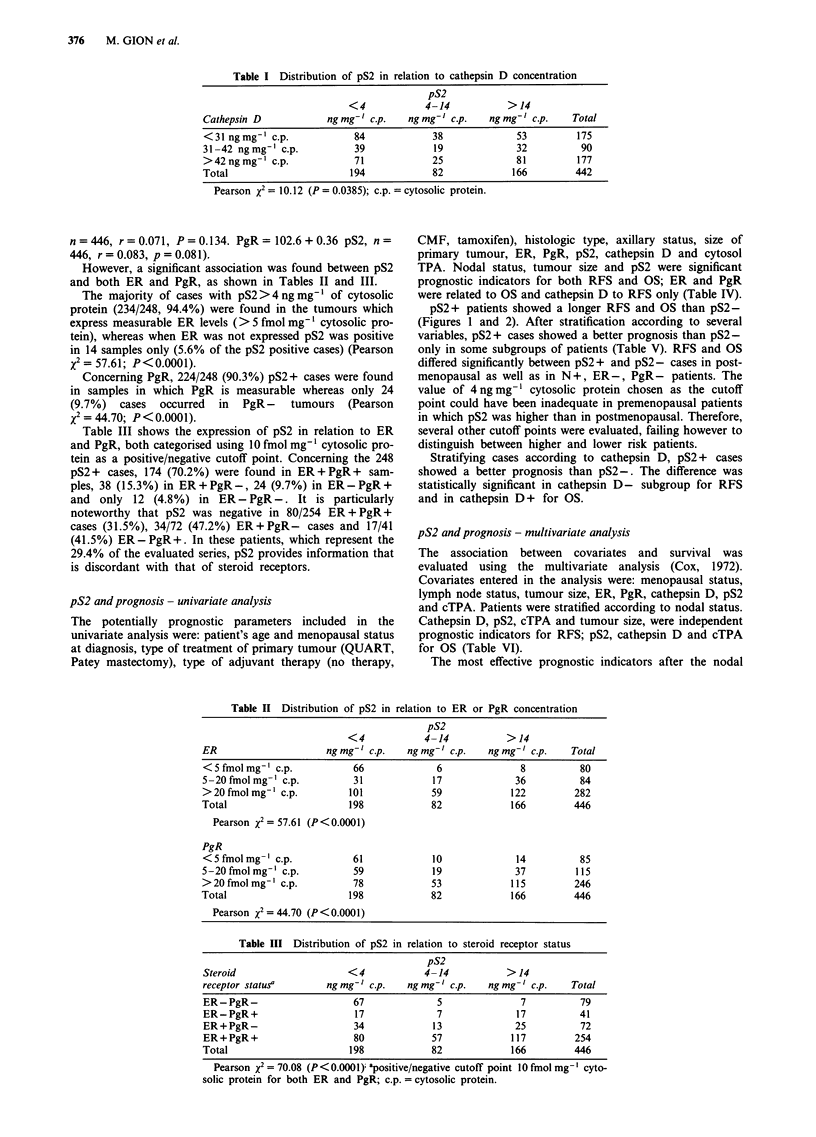
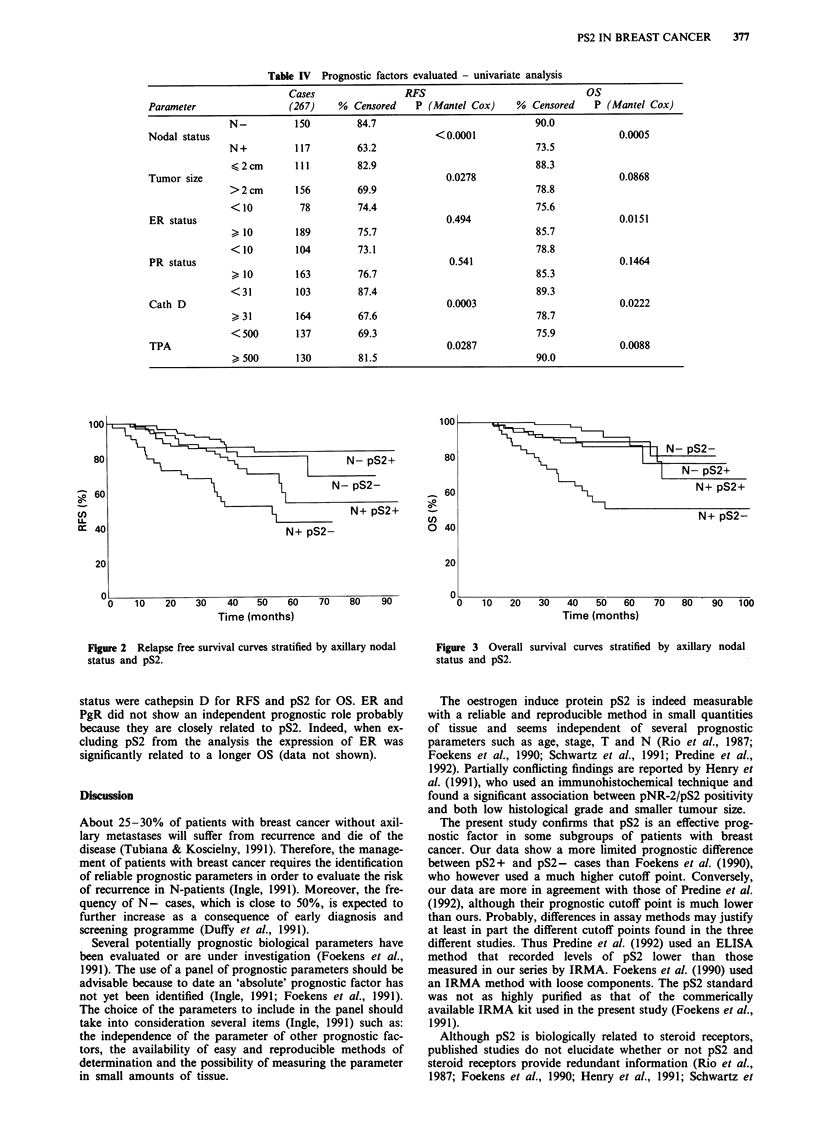
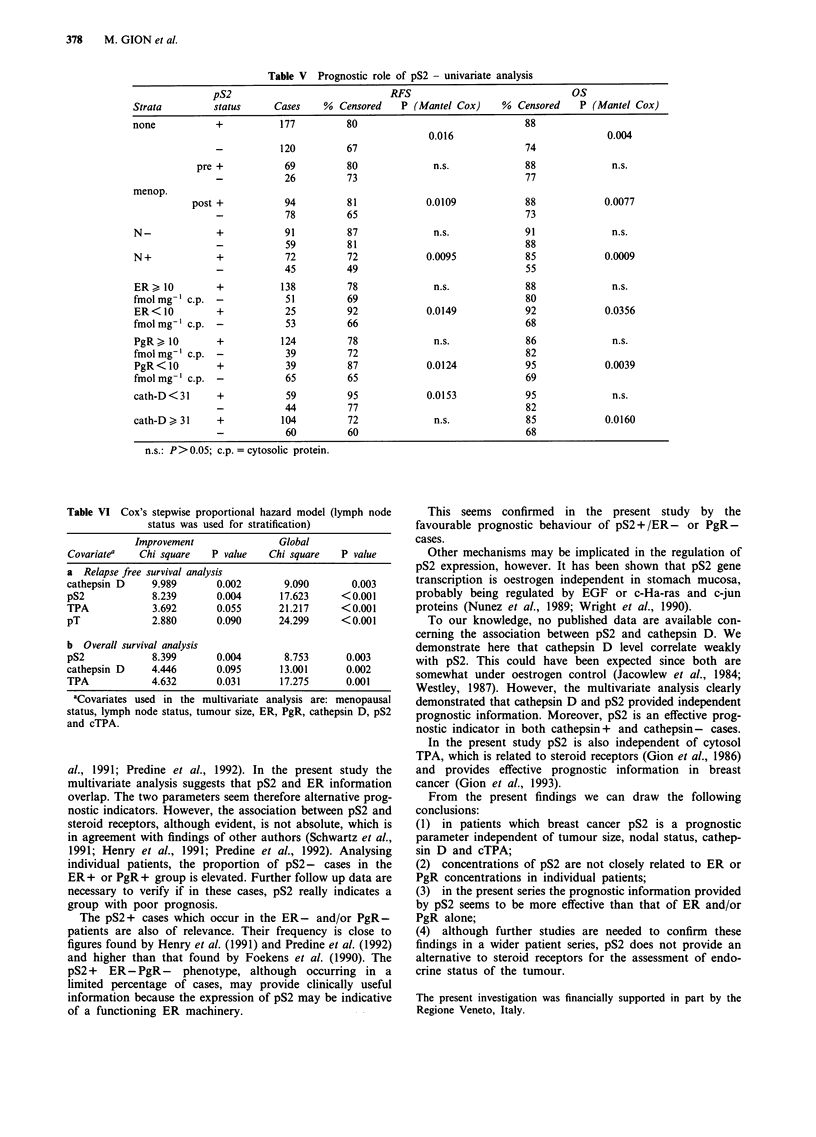
The oestrogen induced pS2 protein was measured in the cytosol of 446 breast cancer samples by an immunoradiometric assay. The relationships between pS2 and several clinical and biological parameters were evaluated. pS2 was not correlated to age, pT and nodal status, while it was higher in pre- than in peri- and post-menopausal women. A statistically significant positive association was found between pS2 and ER, PgR and cathepsin D. However, the frequency of pS2 negative values in ER+ (25.6%), PgR+ (21.7%) and cathepsin D-(19.0%) cases suggests that pS2 provides information independent of the above parameters in a fairly high percentage of patients. The prognostic role of pS2 was evaluated in 267 cases (follow up time 24-102 months). pS2+ showed longer RFS (P = 0.016) and OS (P = 0.004) than pS2-. pS2+ cases were significantly associated with a better prognosis in N+ but not in N- cases. Multivariate analysis showed that pS2 is an independent prognostic factor being the second most effective indicator for OS after nodal status and the third for RFS after nodal status and cathepsin D. From the present findings, we conclude that pS2 probably provides additional biological information to steroid receptor status and cathepsin D in patients with primary breast cancer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltomaa S., Lipponen P., Eskelinen M., Kosma V. M., Marin S., Alhava E., Syrjänen K. Hormone receptors as prognostic factors in female breast cancer. Ann Med. 1991 Dec;23(6):643–648. doi: 10.3109/07853899109148097. [DOI] [PubMed] [Google Scholar]
- Adams D. J., Edwards D. P., McGuire W. L. Estrogen regulation of specific proteins as a mode of hormone action in human breast cancer. Biomembranes. 1983;11:389–414. [PubMed] [Google Scholar]
- Alexieva-Figusch J., Van Putten W. L., Blankenstein M. A., Blonk-Van Der Wijst J., Klijn J. G. The prognostic value and relationships of patient characteristics, estrogen and progestin receptors, and site of relapse in primary breast cancer. Cancer. 1988 Feb 15;61(4):758–768. doi: 10.1002/1097-0142(19880215)61:4<758::aid-cncr2820610421>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Arvan D. A. Tumor cell heterogeneity: an overview. Clin Chim Acta. 1992 Mar 13;206(1-2):3–7. doi: 10.1016/0009-8981(92)90003-9. [DOI] [PubMed] [Google Scholar]
- Clark G. M., McGuire W. L., Hubay C. A., Pearson O. H., Marshall J. S. Progesterone receptors as a prognostic factor in Stage II breast cancer. N Engl J Med. 1983 Dec 1;309(22):1343–1347. doi: 10.1056/nejm198312013092240. [DOI] [PubMed] [Google Scholar]
- Duffy S. W., Tabar L., Fagerberg G., Gad A., Gröntoft O., South M. C., Day N. E. Breast screening, prognostic factors and survival--results from the Swedish two county study. Br J Cancer. 1991 Dec;64(6):1133–1138. doi: 10.1038/bjc.1991.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foekens J. A., Rio M. C., Seguin P., van Putten W. L., Fauque J., Nap M., Klijn J. G., Chambon P. Prediction of relapse and survival in breast cancer patients by pS2 protein status. Cancer Res. 1990 Jul 1;50(13):3832–3837. [PubMed] [Google Scholar]
- Fuqua S. A., Fitzgerald S. D., Chamness G. C., Tandon A. K., McDonnell D. P., Nawaz Z., O'Malley B. W., McGuire W. L. Variant human breast tumor estrogen receptor with constitutive transcriptional activity. Cancer Res. 1991 Jan 1;51(1):105–109. [PubMed] [Google Scholar]
- Gion M., Mione R., Dittadi R., Fasan S., Pallini A., Bruscagnin G. Carcinoembryonic antigen, ferritin, and tissue polypeptide antigen in serum and tissue. Relationship with the receptor content in breast carcinoma. Cancer. 1986 Mar 1;57(5):917–922. doi: 10.1002/1097-0142(19860301)57:5<917::aid-cncr2820570506>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Henry J. A., Piggott N. H., Mallick U. K., Nicholson S., Farndon J. R., Westley B. R., May F. E. pNR-2/pS2 immunohistochemical staining in breast cancer: correlation with prognostic factors and endocrine response. Br J Cancer. 1991 Apr;63(4):615–622. doi: 10.1038/bjc.1991.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Jakowlew S. B., Breathnach R., Jeltsch J. M., Masiakowski P., Chambon P. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984 Mar 26;12(6):2861–2878. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellerick D. M., Osborn M., Weber K. On the nature of serological tissue polypeptide antigen (TPA); monoclonal keratin 8, 18, and 19 antibodies react differently with TPA prepared from human cultured carcinoma cells and TPA in human serum. Oncogene. 1990 Jul;5(7):1007–1017. [PubMed] [Google Scholar]
- Nunez A. M., Berry M., Imler J. L., Chambon P. The 5' flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989 Mar;8(3):823–829. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez A. M., Jakowlev S., Briand J. P., Gaire M., Krust A., Rio M. C., Chambon P. Characterization of the estrogen-induced pS2 protein secreted by the human breast cancer cell line MCF-7. Endocrinology. 1987 Nov;121(5):1759–1765. doi: 10.1210/endo-121-5-1759. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predine J., Spyratos F., Prud'homme J. F., Andrieu C., Hacene K., Brunet M., Pallud C., Milgrom E. Enzyme-linked immunosorbent assay of pS2 in breast cancers, benign tumors, and normal breast tissues. Correlation with prognosis and adjuvant hormone therapy. Cancer. 1992 Apr 15;69(8):2116–2123. doi: 10.1002/1097-0142(19920415)69:8<2116::aid-cncr2820690818>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Rayter Z. Steroid receptors in breast cancer. Br J Surg. 1991 May;78(5):528–535. doi: 10.1002/bjs.1800780506. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Gairard B., Rasmussen U. B., Krust A., Koehl C., Calderoli H., Schiff V., Renaud R., Chambon P. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio M. C., Chambon P. The pS2 gene, mRNA, and protein: a potential marker for human breast cancer. Cancer Cells. 1990 Aug-Sep;2(8-9):269–274. [PubMed] [Google Scholar]
- Schwartz L. H., Koerner F. C., Edgerton S. M., Sawicka J. M., Rio M. C., Bellocq J. P., Chambon P., Thor A. D. pS2 expression and response to hormonal therapy in patients with advanced breast cancer. Cancer Res. 1991 Jan 15;51(2):624–628. [PubMed] [Google Scholar]
- Sluyser M., Wittliff J. L. Influence of estrogen receptor variants in mammary carcinomas on the prognostic reliability of the receptor assay. Mol Cell Endocrinol. 1992 May;85(1-2):83–88. doi: 10.1016/0303-7207(92)90127-r. [DOI] [PubMed] [Google Scholar]
- Spyratos F., Maudelonde T., Brouillet J. P., Brunet M., Defrenne A., Andrieu C., Hacene K., Desplaces A., Rouëssé J., Rochefort H. Cathepsin D: an independent prognostic factor for metastasis of breast cancer. Lancet. 1989 Nov 11;2(8672):1115–1118. doi: 10.1016/s0140-6736(89)91487-6. [DOI] [PubMed] [Google Scholar]
- Tandon A. K., Clark G. M., Chamness G. C., Chirgwin J. M., McGuire W. L. Cathepsin D and prognosis in breast cancer. N Engl J Med. 1990 Feb 1;322(5):297–302. doi: 10.1056/NEJM199002013220504. [DOI] [PubMed] [Google Scholar]
- Thorpe S. M. Estrogen and progesterone receptor determinations in breast cancer. Technology, biology and clinical significance. Acta Oncol. 1988;27(1):1–19. doi: 10.3109/02841868809090312. [DOI] [PubMed] [Google Scholar]
- Tubiana M., Koscielny S. Natural history of human breast cancer: recent data and clinical implications. Breast Cancer Res Treat. 1991 Aug;18(3):125–140. doi: 10.1007/BF01990028. [DOI] [PubMed] [Google Scholar]
- Westley B. R., May F. E. Oestrogen regulates cathepsin D mRNA levels in oestrogen responsive human breast cancer cells. Nucleic Acids Res. 1987 May 11;15(9):3773–3786. doi: 10.1093/nar/15.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]


