Abstract
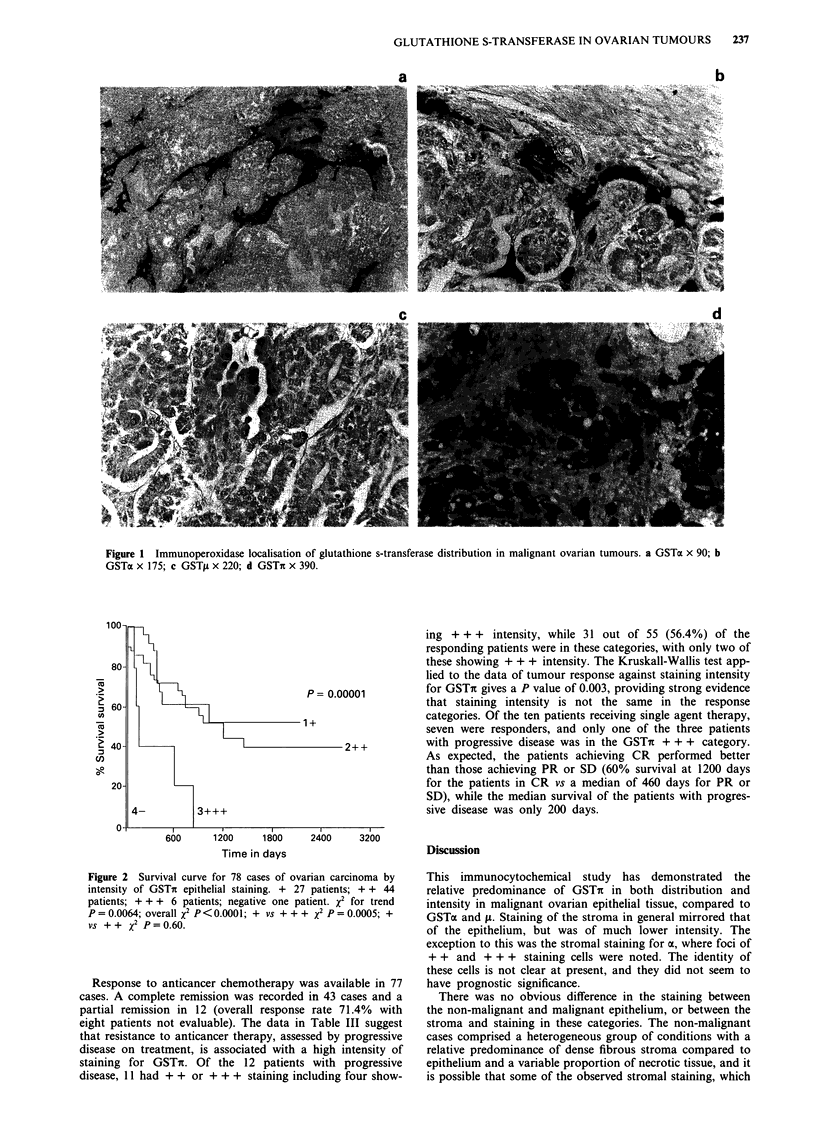
Glutathione S-transferase sub-types alpha, mu and pi were assessed by immunocytochemistry in 109 biopsies of ovarian tissue, comprising malignant epithelial tissue in 86 cases and tissue of ovarian origin considered to be normal in 23. Glutathione S-transferase pi was the most prevalent, being present in all except one malignant epithelium studied and 83% of non-malignant tissue. There were no significant differences in the overall distribution of positive staining for alpha, mu and pi in the malignant and non-malignant biopsies, although the intensity of staining was greater in the malignant epithelium. Stromal staining was in general more pronounced in the malignant biopsies, and this was particularly prominent in the case of the alpha sub-type. Positive staining was seen more frequently in the less well-differentiated tumours, and a diffuse cytoplasmic pattern was the most common observation in tumours of moderate and poor differentiation. There was no significant association between survival and the presence or absence of sub-type staining of alpha and mu sub-type. For the sub-type pi, patient survival was found to correlate with the intensity of staining (on a 0-(+++) scale). Those patients showing resistance to cytotoxic chemotherapy were found to have a higher intensity of staining for GST pi than responding patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black S. M., Beggs J. D., Hayes J. D., Bartoszek A., Muramatsu M., Sakai M., Wolf C. R. Expression of human glutathione S-transferases in Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. Biochem J. 1990 Jun 1;268(2):309–315. doi: 10.1042/bj2680309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. S., Thorner P. S., Haddad G., Ling V. Immunohistochemical detection of P-glycoprotein: prognostic correlation in soft tissue sarcoma of childhood. J Clin Oncol. 1990 Apr;8(4):689–704. doi: 10.1200/JCO.1990.8.4.689. [DOI] [PubMed] [Google Scholar]
- Christen R. D., Hom D. K., Porter D. C., Andrews P. A., MacLeod C. L., Hafstrom L., Howell S. B. Epidermal growth factor regulates the in vitro sensitivity of human ovarian carcinoma cells to cisplatin. J Clin Invest. 1990 Nov;86(5):1632–1640. doi: 10.1172/JCI114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C., Hilton J. Characterization of melphalan-glutathione adducts whose formation is catalyzed by glutathione transferases. Biochem Pharmacol. 1986 Oct 1;35(19):3405–3409. doi: 10.1016/0006-2952(86)90444-2. [DOI] [PubMed] [Google Scholar]
- Evans C. G., Bodell W. J., Tokuda K., Doane-Setzer P., Smith M. T. Glutathione and related enzymes in rat brain tumor cell resistance to 1,3-bis(2-chloroethyl)-1-nitrosourea and nitrogen mustard. Cancer Res. 1987 May 15;47(10):2525–2530. [PubMed] [Google Scholar]
- Farber E., Sarma D. S. Hepatocarcinogenesis: a dynamic cellular perspective. Lab Invest. 1987 Jan;56(1):4–22. [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane J. S., Hird V., Hughes C. M., Gullick W. J. c-erbB-2 oncogene expression in ovarian cancer. J Pathol. 1990 Nov;162(3):231–237. doi: 10.1002/path.1711620309. [DOI] [PubMed] [Google Scholar]
- Holmes J., Wareing C., Jacobs A., Hayes J. D., Padua R. A., Wolf C. R. Glutathione-s-transferase pi expression in leukaemia: a comparative analysis with mdr-1 data. Br J Cancer. 1990 Aug;62(2):209–212. doi: 10.1038/bjc.1990.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith W. N., Stallard S., Brown R. Expression of mdr1 and gst-pi in human breast tumours: comparison to in vitro chemosensitivity. Br J Cancer. 1990 May;61(5):712–716. doi: 10.1038/bjc.1990.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. D., Hayes J. D., Wolf C. R. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis. 1988 Jul;9(7):1283–1287. doi: 10.1093/carcin/9.7.1283. [DOI] [PubMed] [Google Scholar]
- Moscow J. A., Townsend A. J., Cowan K. H. Elevation of pi class glutathione S-transferase activity in human breast cancer cells by transfection of the GST pi gene and its effect on sensitivity to toxins. Mol Pharmacol. 1989 Jul;36(1):22–28. [PubMed] [Google Scholar]
- Nakagawa K., Saijo N., Tsuchida S., Sakai M., Tsunokawa Y., Yokota J., Muramatsu M., Sato K., Terada M., Tew K. D. Glutathione-S-transferase pi as a determinant of drug resistance in transfectant cell lines. J Biol Chem. 1990 Mar 15;265(8):4296–4301. [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Needham G. K., Malcolm A. J., Harris A. L. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987 Jun 20;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- Sato K. Glutathione S-transferases and hepatocarcinogenesis. Jpn J Cancer Res. 1988 May;79(5):556–572. doi: 10.1111/j.1349-7006.1988.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Evans C. G., Doane-Setzer P., Castro V. M., Tahir M. K., Mannervik B. Denitrosation of 1,3-bis(2-chloroethyl)-1-nitrosourea by class mu glutathione transferases and its role in cellular resistance in rat brain tumor cells. Cancer Res. 1989 May 15;49(10):2621–2625. [PubMed] [Google Scholar]
- Teicher B. A., Frei E., 3rd Modulation of antitumor alkylating agents (AA). Cancer Treat Res. 1991;57:261–295. doi: 10.1007/978-1-4615-3872-1_13. [DOI] [PubMed] [Google Scholar]
- Teicher B. A., Holden S. A., Kelley M. J., Shea T. C., Cucchi C. A., Rosowsky A., Henner W. D., Frei E., 3rd Characterization of a human squamous carcinoma cell line resistant to cis-diamminedichloroplatinum(II). Cancer Res. 1987 Jan 15;47(2):388–393. [PubMed] [Google Scholar]
- Wang A. L., Tew K. D. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep. 1985 Jun;69(6):677–682. [PubMed] [Google Scholar]