Abstract
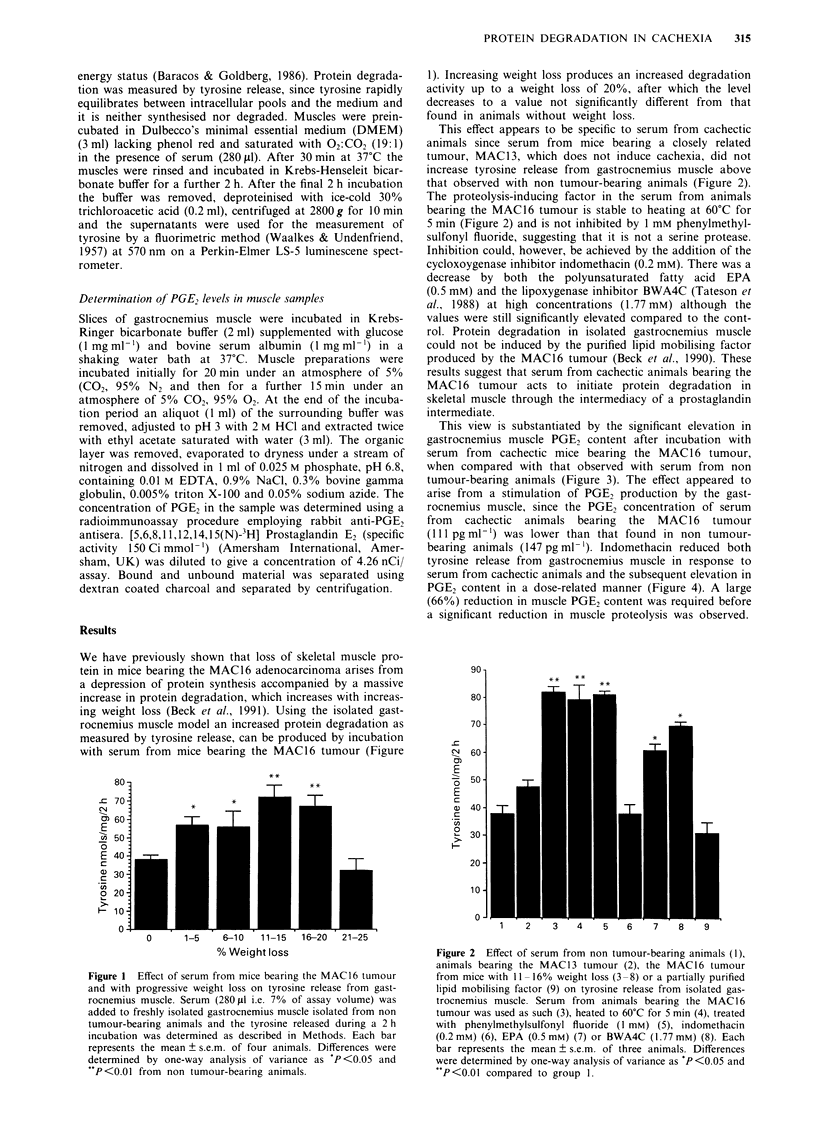
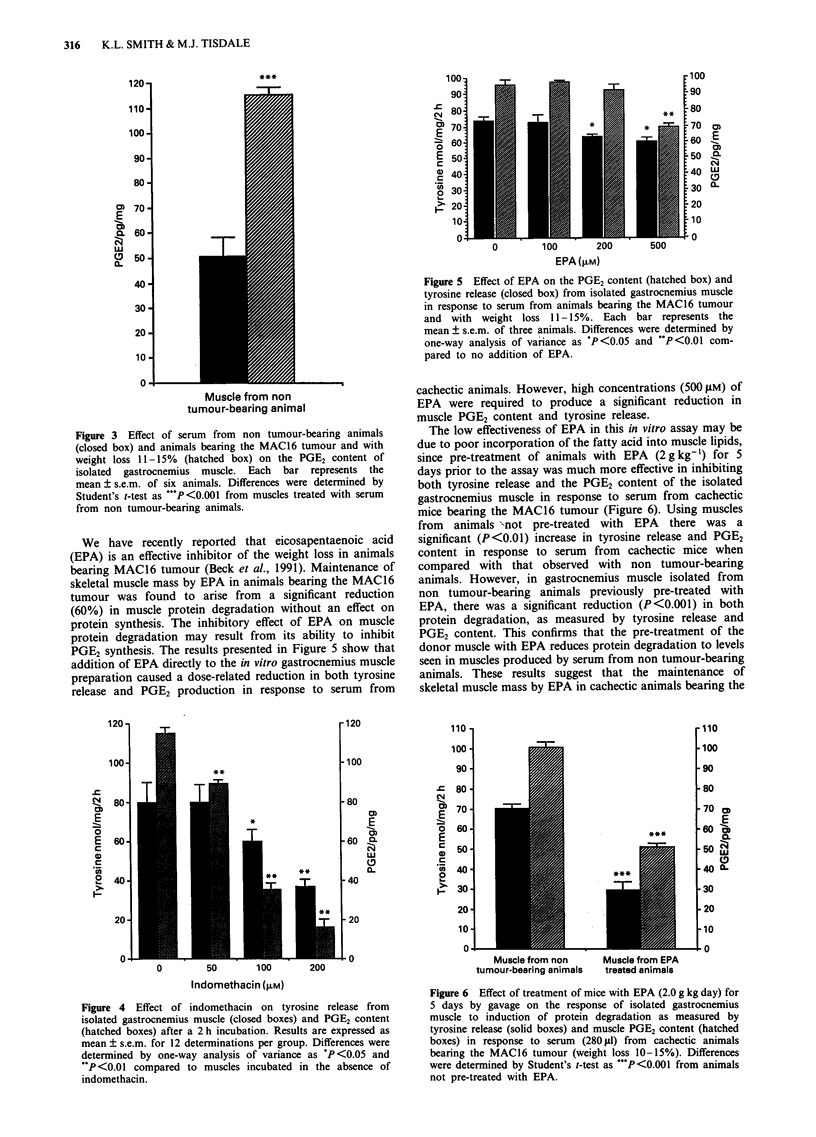
Depletion of skeletal muscle mass in animals bearing an experimental model of cachexia, the MAC16 adenocarcinoma, occurs by a reduction in protein synthesis accompanied by a large increase in protein degradation. Serum from mice bearing the MAC16 tumour produced an increased protein degradation in isolated gastrocnemius muscle, as measured by tyrosine release, with a maximal effect occurring with serum from animals with a weight loss of between 11 and 20%. The response was specific to the cachectic state, since serum from mice bearing the MAC13 adenocarcinoma, which does not produce weight loss, did not increase tyrosine release from gastrocnemius muscle above that observed with serum from non tumour-bearing animals. The circulatory proteolysis-inducing factor was stable to heating at 60 degrees C for 5 min and was not inhibited by phenylmethylsulfonyl fluoride, suggesting that it was not a serine protease. The level of prostaglandin E2 (PGE2) in gastrocnemius muscle was significantly elevated after incubation with serum from cachectic mice bearing the MAC16 tumour. Both indomethacin and the polyunsaturated fatty acid eicosapentaenoic acid (EPA) inhibited the rise in muscle PGE2 content in response to serum from cachectic mice and also inhibited muscle protein degradation. These results suggest that muscle protein degradation in cancer cachexia is associated with a rise in PGE2 content.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V. E., Goldberg A. L. Maintenance of normal length improves protein balance and energy status in isolated rat skeletal muscles. Am J Physiol. 1986 Oct;251(4 Pt 1):C588–C596. doi: 10.1152/ajpcell.1986.251.4.C588. [DOI] [PubMed] [Google Scholar]
- Beck S. A., Mulligan H. D., Tisdale M. J. Lipolytic factors associated with murine and human cancer cachexia. J Natl Cancer Inst. 1990 Dec 19;82(24):1922–1926. doi: 10.1093/jnci/82.24.1922. [DOI] [PubMed] [Google Scholar]
- Beck S. A., Smith K. L., Tisdale M. J. Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res. 1991 Nov 15;51(22):6089–6093. [PubMed] [Google Scholar]
- Beck S. A., Tisdale M. J. Production of lipolytic and proteolytic factors by a murine tumor-producing cachexia in the host. Cancer Res. 1987 Nov 15;47(22):5919–5923. [PubMed] [Google Scholar]
- Belizario J. E., Katz M., Chenker E., Raw I. Bioactivity of skeletal muscle proteolysis-inducing factors in the plasma proteins from cancer patients with weight loss. Br J Cancer. 1991 May;63(5):705–710. doi: 10.1038/bjc.1991.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby M. C., Double J. A., Ali S. A., Fearon K. C., Brennan R. A., Tisdale M. J. Characterization of a transplantable adenocarcinoma of the mouse colon producing cachexia in recipient animals. J Natl Cancer Inst. 1987 Mar;78(3):539–546. [PubMed] [Google Scholar]
- Flores E. A., Bistrian B. R., Pomposelli J. J., Dinarello C. A., Blackburn G. L., Istfan N. W. Infusion of tumor necrosis factor/cachectin promotes muscle catabolism in the rat. A synergistic effect with interleukin 1. J Clin Invest. 1989 May;83(5):1614–1622. doi: 10.1172/JCI114059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groundwater P., Beck S. A., Barton C., Adamson C., Ferrier I. N., Tisdale M. J. Alteration of serum and urinary lipolytic activity with weight loss in cachectic cancer patients. Br J Cancer. 1990 Nov;62(5):816–821. doi: 10.1038/bjc.1990.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstein M. K., Meydani S. N., Meydani M., Wu K., Dinarello C. A. Interleukin-1-induced anorexia in the rat. Influence of prostaglandins. J Clin Invest. 1989 Jul;84(1):228–235. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallinowski F., Runkel S., Fortmeyer H. P., Förster H., Vaupel P. L-glutamine: a major substrate for tumor cells in vivo? J Cancer Res Clin Oncol. 1987;113(3):209–215. doi: 10.1007/BF00396375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo P. A. Tumour induction of host leucine starvation. FEBS Lett. 1981 Dec 7;135(2):229–231. doi: 10.1016/0014-5793(81)80788-0. [DOI] [PubMed] [Google Scholar]
- Levine L., Worth N. Eicosapentaenoic acid: its effects on arachidonic acid metabolism by cells in culture. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 2):430–436. doi: 10.1016/0091-6749(84)90143-x. [DOI] [PubMed] [Google Scholar]
- Lundholm K., Edström S., Ekman L., Karlberg I., Bylund A. C., Scherstén T. A comparative study of the influence of malignant tumor on host metabolism in mice and man: evaluation of an experimental model. Cancer. 1978 Aug;42(2):453–461. doi: 10.1002/1097-0142(197808)42:2<453::aid-cncr2820420212>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Moldawer L. L., Svaninger G., Gelin J., Lundholm K. G. Interleukin 1 and tumor necrosis factor do not regulate protein balance in skeletal muscle. Am J Physiol. 1987 Dec;253(6 Pt 1):C766–C773. doi: 10.1152/ajpcell.1987.253.6.C766. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Stein T. P. Cachexia, gluconeogenesis and progressive weight loss in cancer patients. J Theor Biol. 1978 Jul 6;73(1):51–59. doi: 10.1016/0022-5193(78)90179-0. [DOI] [PubMed] [Google Scholar]
- Strelkov A. B., Fields A. L., Baracos V. E. Effects of systemic inhibition of prostaglandin production on protein metabolism in tumor-bearing rats. Am J Physiol. 1989 Aug;257(2 Pt 1):C261–C269. doi: 10.1152/ajpcell.1989.257.2.C261. [DOI] [PubMed] [Google Scholar]
- Tateson J. E., Randall R. W., Reynolds C. H., Jackson W. P., Bhattacherjee P., Salmon J. A., Garland L. G. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: biochemical assessment in vitro and ex vivo. Br J Pharmacol. 1988 Jun;94(2):528–539. doi: 10.1111/j.1476-5381.1988.tb11557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale M. J., Beck S. A. Inhibition of tumour-induced lipolysis in vitro and cachexia and tumour growth in vivo by eicosapentaenoic acid. Biochem Pharmacol. 1991 Jan 1;41(1):103–107. doi: 10.1016/0006-2952(91)90016-x. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Wu G. Y., Thompson J. R. The effect of ketone bodies on alanine and glutamine metabolism in isolated skeletal muscle from the fasted chick. Biochem J. 1988 Oct 1;255(1):139–144. doi: 10.1042/bj2550139. [DOI] [PMC free article] [PubMed] [Google Scholar]



