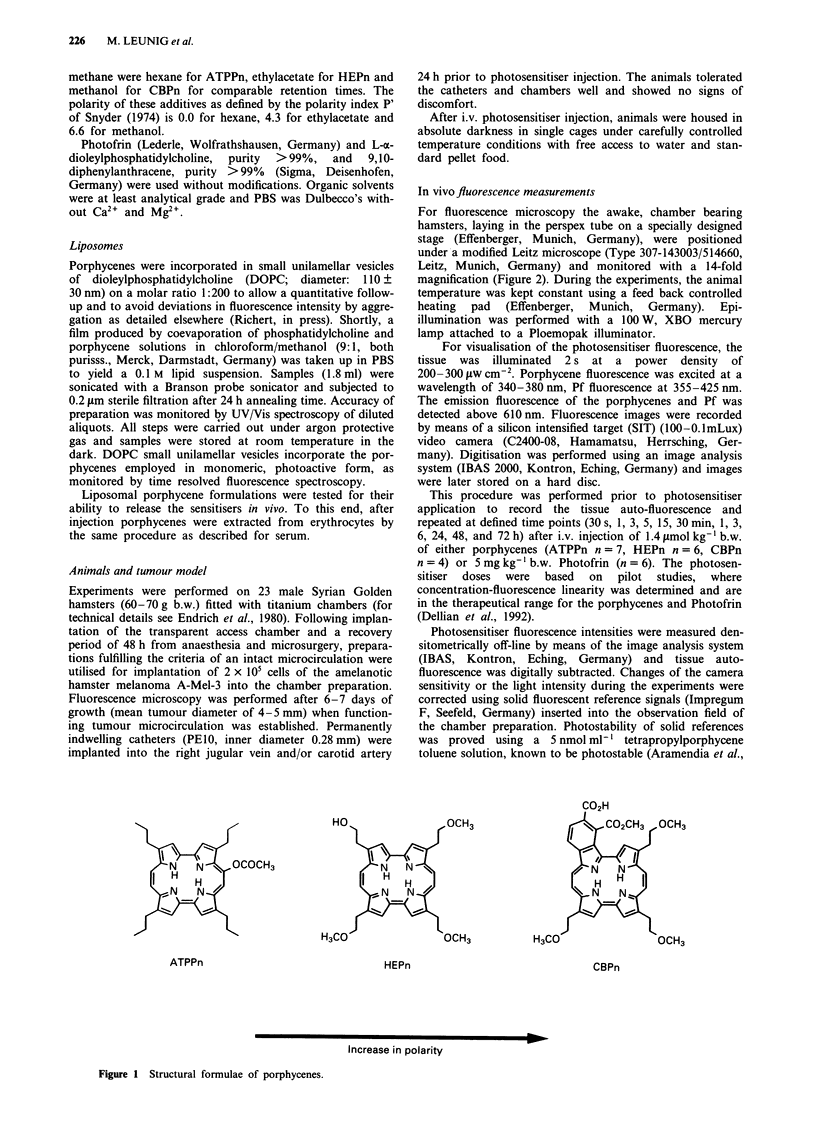
Abstract
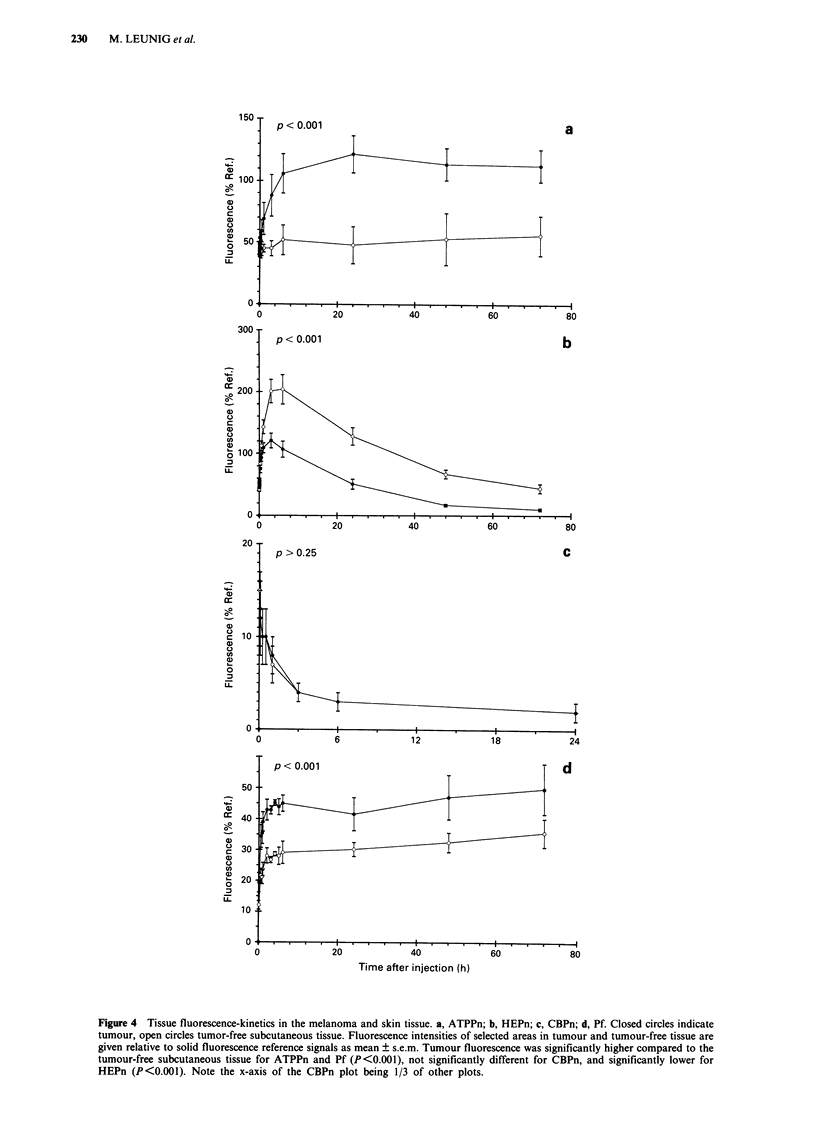
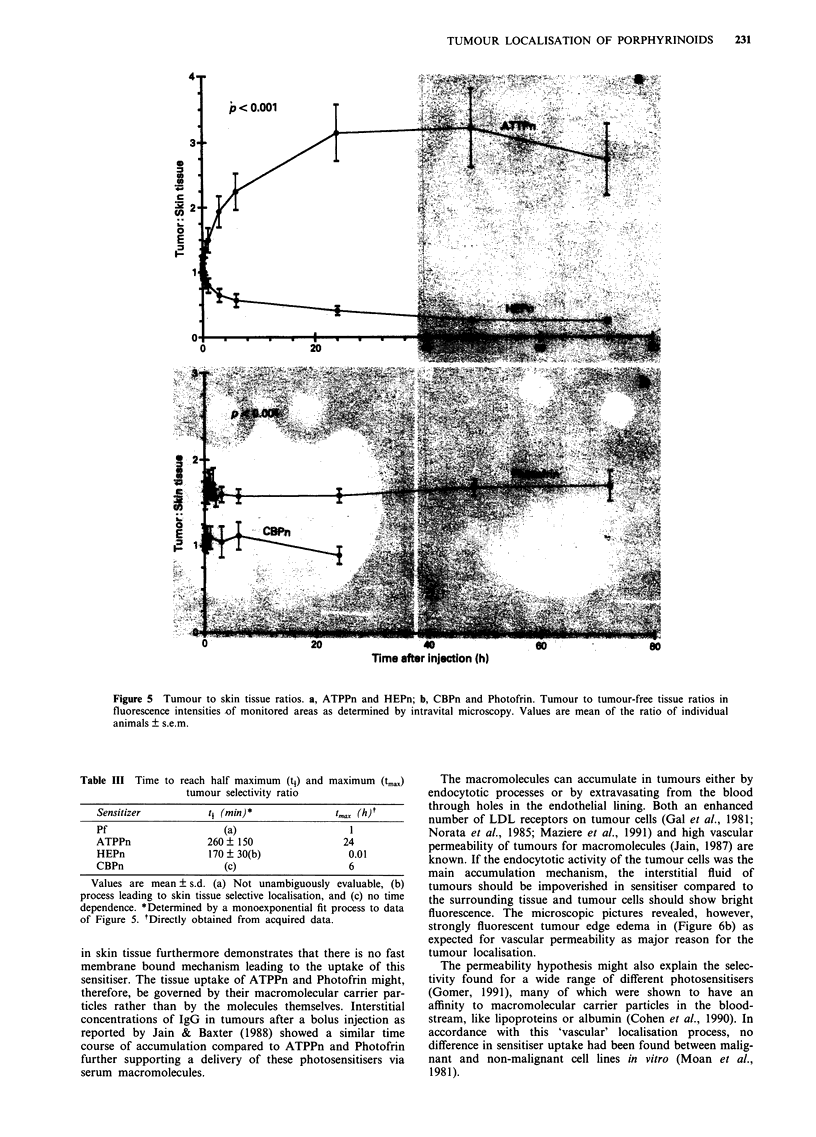
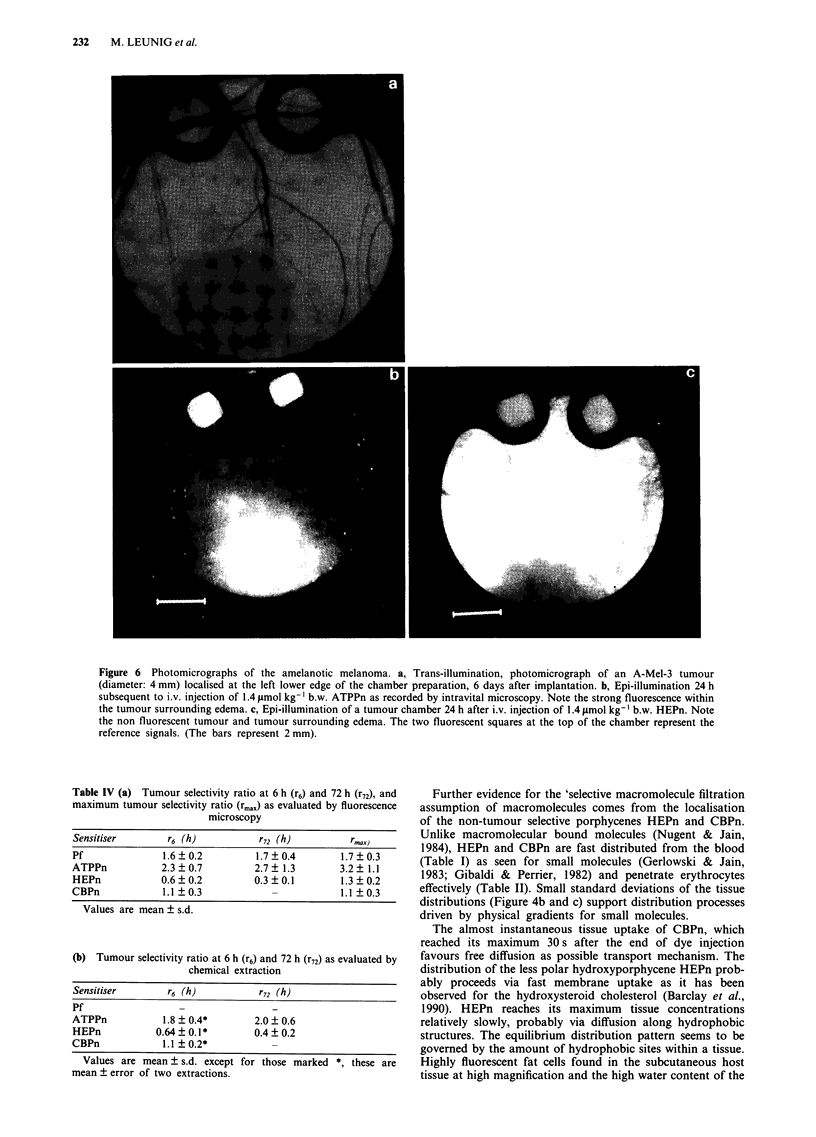
In this study the localisation of porphyrinoid photosensitizers in tumours was investigated. To determine if tumour selectivity results from a preferential uptake or prolonged retention of photosensitizers, intravital fluorescence microscopy and chemical extraction were used. Amelanotic melanoma (A-Mel-3) were implanted in a skin fold chamber in Syrian Golden hamsters. Distribution of the porphyrin mixture Photofrin and three porphycenes, pure porphyrinoid model compounds, was studied quantitatively by intravital fluorescence microscopy. Extraction of tissue and blood samples was performed to verify and supplement intravital microscopic results. Photofrin accumulated in melanomas reaching a maximum tumour:skin tissue ratio of 1.7:1. Localisation of the different porphycenes was found to be highly tumour selective (3.2:1), anti-tumour selective (0.2:1), and non-selective (1:1) with increasing polarity of the porphycenes. The two non-tumour selective porphycenes had distinctly accelerated serum and tissue kinetics; serum halflife times being as short as 1 min. The specific localisation of the slowly distributed, tumour selective photosensitizers, occurred exclusively during the distribution from serum and uptake into tissues. For the most selective porphycene, the tumour selection process had a halflife of 260 +/- 150 min and led to a strongly fluorescent tumour edge edema. Accumulation of porphyrines by the amelanotic melanoma (A-Mel-3) can be attributed to an enhanced uptake rate for lipophilic molecules in this subcutaneously growing neoplasm. The slow distribution of the two tumour specific photosensitizers and the strong fluorescence of these hydrophobic molecules in the tumour compartment with a high water content indicate a carrier role of serum proteins in the selection process. Enhanced permeability of the tumour vasculature to macromolecules appears to be the most probable reason for the tumour selectivity of these two sensitisers.
Full text
PDF
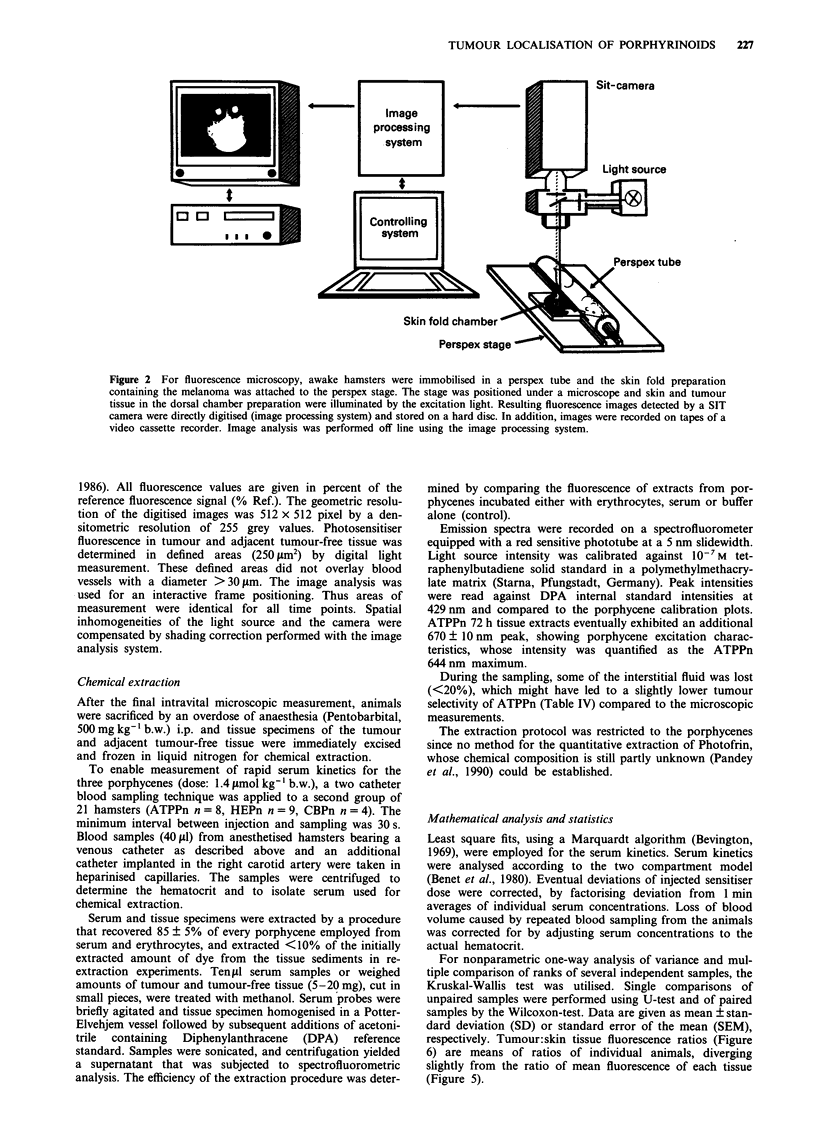
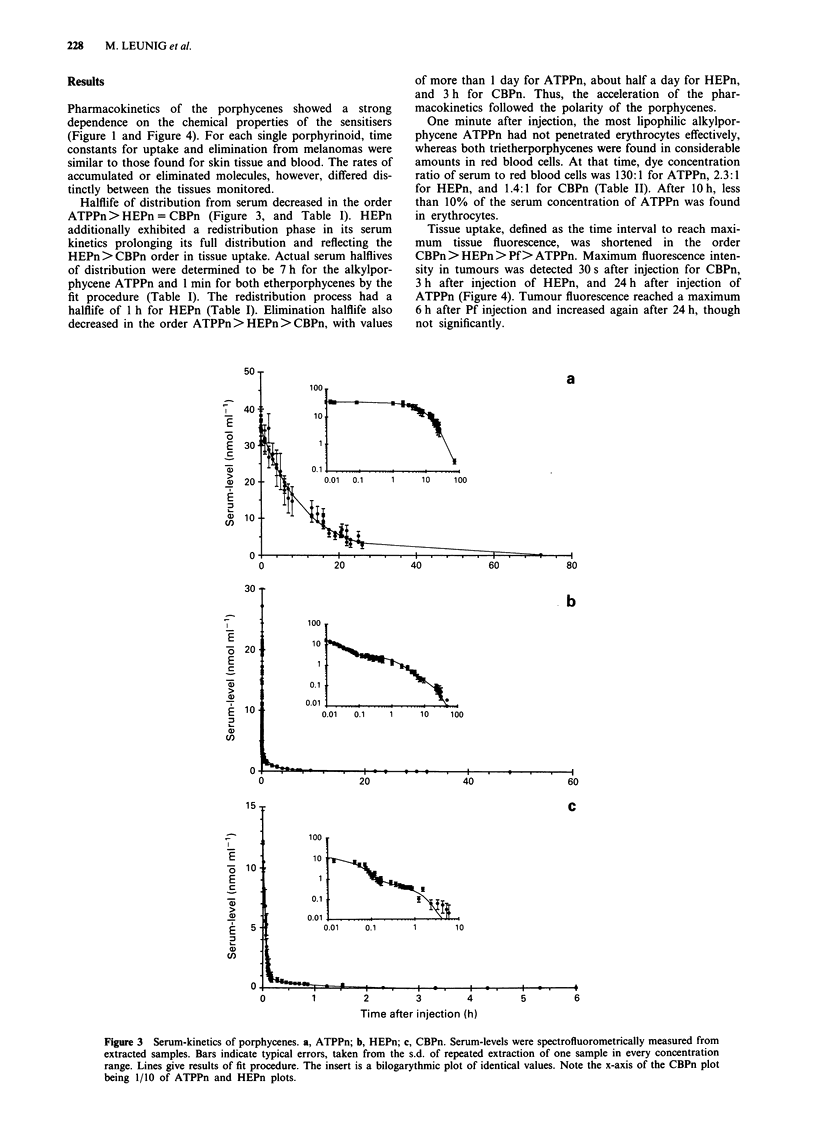



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aramendia P. F., Redmond R. W., Nonell S., Schuster W., Braslavsky S. E., Schaffner K., Vogel E. The photophysical properties of porphycenes: potential photodynamic therapy agents. Photochem Photobiol. 1986 Nov;44(5):555–559. doi: 10.1111/j.1751-1097.1986.tb04708.x. [DOI] [PubMed] [Google Scholar]
- Asaishi K., Endrich B., Götz A., Messmer K. Quantitative analysis of microvascular structure and function in the amelanotic melanoma A-Mel-3. Cancer Res. 1981 May;41(5):1898–1904. [PubMed] [Google Scholar]
- Barclay L. R., Cameron R. C., Forrest B. J., Locke S. J., Nigam R., Vinqvist M. R. Cholesterol: free radical peroxidation and transfer into phospholipid membranes. Biochim Biophys Acta. 1990 Dec 4;1047(3):255–263. doi: 10.1016/0005-2760(90)90524-2. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kennedy J. C., Jones R. A., Nadeau P., Pottier R. H. The effect of tissue and cellular pH on the selective biodistribution of porphyrin-type photochemotherapeutic agents: a volumetric titration study. J Photochem Photobiol B. 1990 Jul;6(3):309–323. doi: 10.1016/1011-1344(90)85101-2. [DOI] [PubMed] [Google Scholar]
- Baumgartner R., Fisslinger H., Jocham D., Lenz H., Ruprecht L., Stepp H., Unsöld E. A fluorescence imaging device for endoscopic detection of early stage cancer--instrumental and experimental studies. Photochem Photobiol. 1987 Nov;46(5):759–763. doi: 10.1111/j.1751-1097.1987.tb04844.x. [DOI] [PubMed] [Google Scholar]
- Bellnier D. A., Ho Y. K., Pandey R. K., Missert J. R., Dougherty T. J. Distribution and elimination of Photofrin II in mice. Photochem Photobiol. 1989 Aug;50(2):221–228. doi: 10.1111/j.1751-1097.1989.tb04152.x. [DOI] [PubMed] [Google Scholar]
- Brault D., Vever-Bizet C., Le Doan T. Spectrofluorimetric study of porphyrin incorporation into membrane models--evidence for pH effects. Biochim Biophys Acta. 1986 May 28;857(2):238–250. doi: 10.1016/0005-2736(86)90352-4. [DOI] [PubMed] [Google Scholar]
- Cohen S., Margalit R. Binding of porphyrin to human serum albumin. Structure-activity relationships. Biochem J. 1990 Sep 1;270(2):325–330. doi: 10.1042/bj2700325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Boyle D. G., Weishaupt K. R., Henderson B. A., Potter W. R., Bellnier D. A., Wityk K. E. Photoradiation therapy--clinical and drug advances. Adv Exp Med Biol. 1983;160:3–13. doi: 10.1007/978-1-4684-4406-3_2. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Kaufman J. E., Goldfarb A., Weishaupt K. R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978 Aug;38(8):2628–2635. [PubMed] [Google Scholar]
- Dougherty T. J. Studies on the structure of porphyrins contained in Photofrin II. Photochem Photobiol. 1987 Nov;46(5):569–573. doi: 10.1111/j.1751-1097.1987.tb04815.x. [DOI] [PubMed] [Google Scholar]
- Endrich B., Asaishi K., Götz A., Messmer K. Technical report--a new chamber technique for microvascular studies in unanesthetized hamsters. Res Exp Med (Berl) 1980;177(2):125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- Endrich B., Hammersen F., Götz A., Messmer K. Microcirculatory blood flow, capillary morphology and local oxygen pressure of the hamster amelanotic melanoma A-Mel-3. J Natl Cancer Inst. 1982 Mar;68(3):475–485. [PubMed] [Google Scholar]
- Freitas I. Lipid accumulation: the common feature to photosensitizer-retaining normal and malignant tissues. J Photochem Photobiol B. 1990 Nov;7(2-4):359–361. doi: 10.1016/1011-1344(90)85169-w. [DOI] [PubMed] [Google Scholar]
- Gal D., MacDonald P. C., Porter J. C., Simpson E. R. Cholesterol metabolism in cancer cells in monolayer culture. III. Low-density lipoprotein metabolism. Int J Cancer. 1981 Sep 15;28(3):315–319. doi: 10.1002/ijc.2910280310. [DOI] [PubMed] [Google Scholar]
- Gerlowski L. E., Jain R. K. Physiologically based pharmacokinetic modeling: principles and applications. J Pharm Sci. 1983 Oct;72(10):1103–1127. doi: 10.1002/jps.2600721003. [DOI] [PubMed] [Google Scholar]
- Gomer C. J. Preclinical examination of first and second generation photosensitizers used in photodynamic therapy. Photochem Photobiol. 1991 Dec;54(6):1093–1107. doi: 10.1111/j.1751-1097.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Guardiano M., Biolo R., Jori G., Schaffner K. Tetra-n-propylporphycene as a tumour localizer: pharmacokinetic and phototherapeutic studies in mice. Cancer Lett. 1989 Jan;44(1):1–6. doi: 10.1016/0304-3835(89)90100-6. [DOI] [PubMed] [Google Scholar]
- Jain R. K., Baxter L. T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988 Dec 15;48(24 Pt 1):7022–7032. [PubMed] [Google Scholar]
- Jain R. K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6(4):559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- Kessel D., Chou T. H. Tumor-localizing components of the porphyrin preparation hematoporphyrin derivative. Cancer Res. 1983 May;43(5):1994–1999. [PubMed] [Google Scholar]
- Kreimer-Birnbaum M. Modified porphyrins, chlorins, phthalocyanines, and purpurins: second-generation photosensitizers for photodynamic therapy. Semin Hematol. 1989 Apr;26(2):157–173. [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J., OLSEN A. M. Hematoporphyrin derivative: a new aid for endoscopic detection of malignant disease. J Thorac Cardiovasc Surg. 1961 Nov;42:623–629. [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J., OLSEN A. M. The use of a derivative of hematoporhyrin in tumor detection. J Natl Cancer Inst. 1961 Jan;26:1–11. [PubMed] [Google Scholar]
- Leunig M., Yuan F., Menger M. D., Boucher Y., Goetz A. E., Messmer K., Jain R. K. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992 Dec 1;52(23):6553–6560. [PubMed] [Google Scholar]
- Mazière J. C., Morlière P., Santus R. The role of the low density lipoprotein receptor pathway in the delivery of lipophilic photosensitizers in the photodynamic therapy of tumours. J Photochem Photobiol B. 1991 Mar;8(4):351–360. doi: 10.1016/1011-1344(91)80111-t. [DOI] [PubMed] [Google Scholar]
- Moan J., Berg K. Photochemotherapy of cancer: experimental research. Photochem Photobiol. 1992 Jun;55(6):931–948. doi: 10.1111/j.1751-1097.1992.tb08541.x. [DOI] [PubMed] [Google Scholar]
- Moan J., Steen H. B., Feren K., Christensen T. Uptake of hematoporphyrin derivative and sensitized photoinactivation of C3H cells with different oncogenic potential. Cancer Lett. 1981 Dec;14(3):291–296. doi: 10.1016/0304-3835(81)90157-9. [DOI] [PubMed] [Google Scholar]
- Norata G., Canti G., Ricci L., Nicolin A., Trezzi E., Catapano A. L. In vivo assimilation of low density lipoproteins by a fibrosarcoma tumour line in mice. Cancer Lett. 1984 Dec;25(2):203–208. doi: 10.1016/s0304-3835(84)80046-4. [DOI] [PubMed] [Google Scholar]
- Nugent L. J., Jain R. K. Plasma pharmacokinetics and interstitial diffusion of macromolecules in a capillary bed. Am J Physiol. 1984 Jan;246(1 Pt 2):H129–H137. doi: 10.1152/ajpheart.1984.246.1.H129. [DOI] [PubMed] [Google Scholar]
- Pandey R. K., Siegel M. M., Tsao R., McReynolds J. H., Dougherty T. J. Fast atom bombardment mass spectral analyses of Photofrin II and its synthetic analogs. Biomed Environ Mass Spectrom. 1990 Jul;19(7):405–414. doi: 10.1002/bms.1200190705. [DOI] [PubMed] [Google Scholar]