Abstract
PCR-based microsatellite polymorphisms have proved their power in genetic linkage analysis and other identification methods, due to their high information content and even distribution over the chromosomes. In the present study we applied microsatellite polymorphisms to detect loss of heterozygosity in fresh (snap-frozen) and in archival ovarian tumour tissue. Clear allele losses were found in fresh and paraffin embedded tumour samples. Conventional Southern analysis of flanking markers on the same tumour DNA samples confirmed the observed losses detected by microsatellite polymorphisms. Titration experiments suggest that loss of heterozygosity remains detectable in tumour samples despite 60% contamination with normal DNA. This technique provides a fast and reproducible alternative to conventional Southern blotting in the detection of loss of heterozygosity, with the crucial additional advantages of minimal sample requirements, making archival material available for genetic investigation.
Full text
PDF
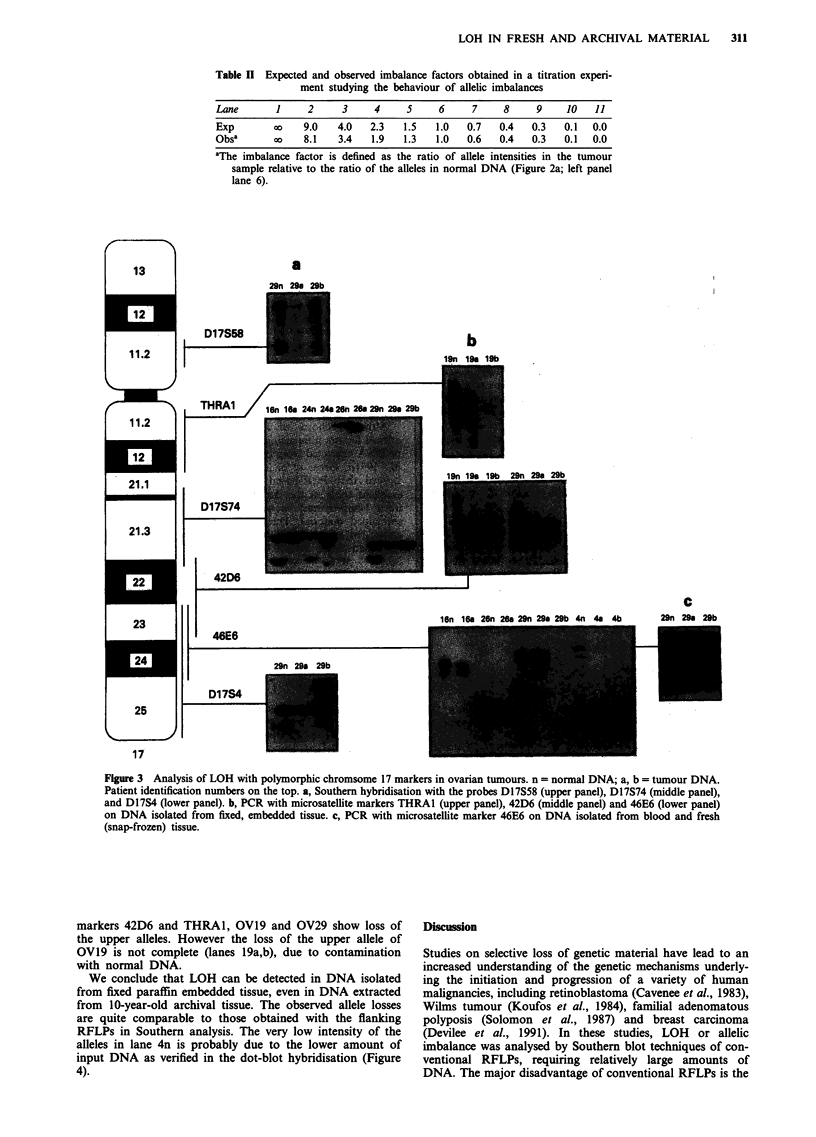


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D., Wright E., Nguyen K., Cannon L., Fain P., Goldgar D., Bishop D. T., Carey J., Baty B., Kivlin J. Gene for von Recklinghausen neurofibromatosis is in the pericentromeric region of chromosome 17. Science. 1987 May 29;236(4805):1100–1102. doi: 10.1126/science.3107130. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Xiang K. S., Newman M. V., Wu S. H., Wright L. G., Fajans S. S., Spielman R. S., Cox N. J. Gene for non-insulin-dependent diabetes mellitus (maturity-onset diabetes of the young subtype) is linked to DNA polymorphism on human chromosome 20q. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1484–1488. doi: 10.1073/pnas.88.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ezra J., Johnson D. A., Rossi J., Cook N., Wu A. Effect of fixation on the amplification of nucleic acids from paraffin-embedded material by the polymerase chain reaction. J Histochem Cytochem. 1991 Mar;39(3):351–354. doi: 10.1177/39.3.1704393. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Caskey C. T. Disease diagnosis by recombinant DNA methods. Science. 1987 Jun 5;236(4806):1223–1229. doi: 10.1126/science.3296189. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger P. L., Schmid C. W. A study of the evolution of repeated DNA sequences in primates and the existence of a new class of repetitive sequences in primates. J Mol Biol. 1979 Feb 5;127(4):437–460. doi: 10.1016/0022-2836(79)90231-6. [DOI] [PubMed] [Google Scholar]
- Devilee P., van Vliet M., Bardoel A., Kievits T., Kuipers-Dijkshoorn N., Pearson P. L., Cornelisse C. J. Frequent somatic imbalance of marker alleles for chromosome 1 in human primary breast carcinoma. Cancer Res. 1991 Feb 1;51(3):1020–1025. [PubMed] [Google Scholar]
- Devilee P., van den Broek M., Kuipers-Dijkshoorn N., Kolluri R., Khan P. M., Pearson P. L., Cornelisse C. J. At least four different chromosomal regions are involved in loss of heterozygosity in human breast carcinoma. Genomics. 1989 Oct;5(3):554–560. doi: 10.1016/0888-7543(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Foulkes W., Black D., Solomon E., Trowsdale J. Allele loss on chromosome 17q in sporadic ovarian cancer. Lancet. 1991 Aug 17;338(8764):444–445. doi: 10.1016/0140-6736(91)91065-3. [DOI] [PubMed] [Google Scholar]
- Greer C. E., Lund J. K., Manos M. M. PCR amplification from paraffin-embedded tissues: recommendations on fixatives for long-term storage and prospective studies. PCR Methods Appl. 1991 Aug;1(1):46–50. doi: 10.1101/gr.1.1.46. [DOI] [PubMed] [Google Scholar]
- Greer C. E., Peterson S. L., Kiviat N. B., Manos M. M. PCR amplification from paraffin-embedded tissues. Effects of fixative and fixation time. Am J Clin Pathol. 1991 Feb;95(2):117–124. doi: 10.1093/ajcp/95.2.117. [DOI] [PubMed] [Google Scholar]
- Hanzlik A. J., Binder M., Layton W. M., Rowe L., Layton M., Taylor B. A., Osemlak M. M., Richards J. E., Kurnit D. M., Stewart G. D. The murine situs inversus viscerum (iv) gene responsible for visceral asymmetry is linked tightly to the Igh-C cluster on chromosome 12. Genomics. 1990 Jul;7(3):389–393. doi: 10.1016/0888-7543(90)90173-r. [DOI] [PubMed] [Google Scholar]
- Jabs E. W., Li X., Coss C. A., Taylor E. W., Meyers D. A., Weber J. L. Mapping the Treacher Collins syndrome locus to 5q31.3----q33.3. Genomics. 1991 Sep;11(1):193–198. [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Louis D. N., von Deimling A., Seizinger B. R. A (CA)n dinucleotide repeat assay for evaluating loss of allelic heterozygosity in small and archival human brain tumor specimens. Am J Pathol. 1992 Oct;141(4):777–782. [PMC free article] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Rose E. A. Applications of the polymerase chain reaction to genome analysis. FASEB J. 1991 Jan;5(1):46–54. doi: 10.1096/fasebj.5.1.1991584. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets H. J., Brunner H. G., Ropers H. H., Wieringa B. Use of variable simple sequence motifs as genetic markers: application to study of myotonic dystrophy. Hum Genet. 1989 Oct;83(3):245–251. doi: 10.1007/BF00285165. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Easton D. F., Evans D. G., Ponder B. A. Allele losses in the region 17q12-21 in familial breast and ovarian cancer involve the wild-type chromosome. Nat Genet. 1992 Oct;2(2):128–131. doi: 10.1038/ng1092-128. [DOI] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Tautz D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989 Aug 25;17(16):6463–6471. doi: 10.1093/nar/17.16.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toguchida J., Ishizaki K., Nakamura Y., Sasaki M. S., Ikenaga M., Kato M., Sugimoto M., Kotoura Y., Yamamuro T. Assignment of common allele loss in osteosarcoma to the subregion 17p13. Cancer Res. 1989 Nov 15;49(22):6247–6251. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Wijmenga C., Frants R. R., Brouwer O. F., Moerer P., Weber J. L., Padberg G. W. Location of facioscapulohumeral muscular dystrophy gene on chromosome 4. Lancet. 1990 Sep 15;336(8716):651–653. doi: 10.1016/0140-6736(90)92148-b. [DOI] [PubMed] [Google Scholar]