Abstract
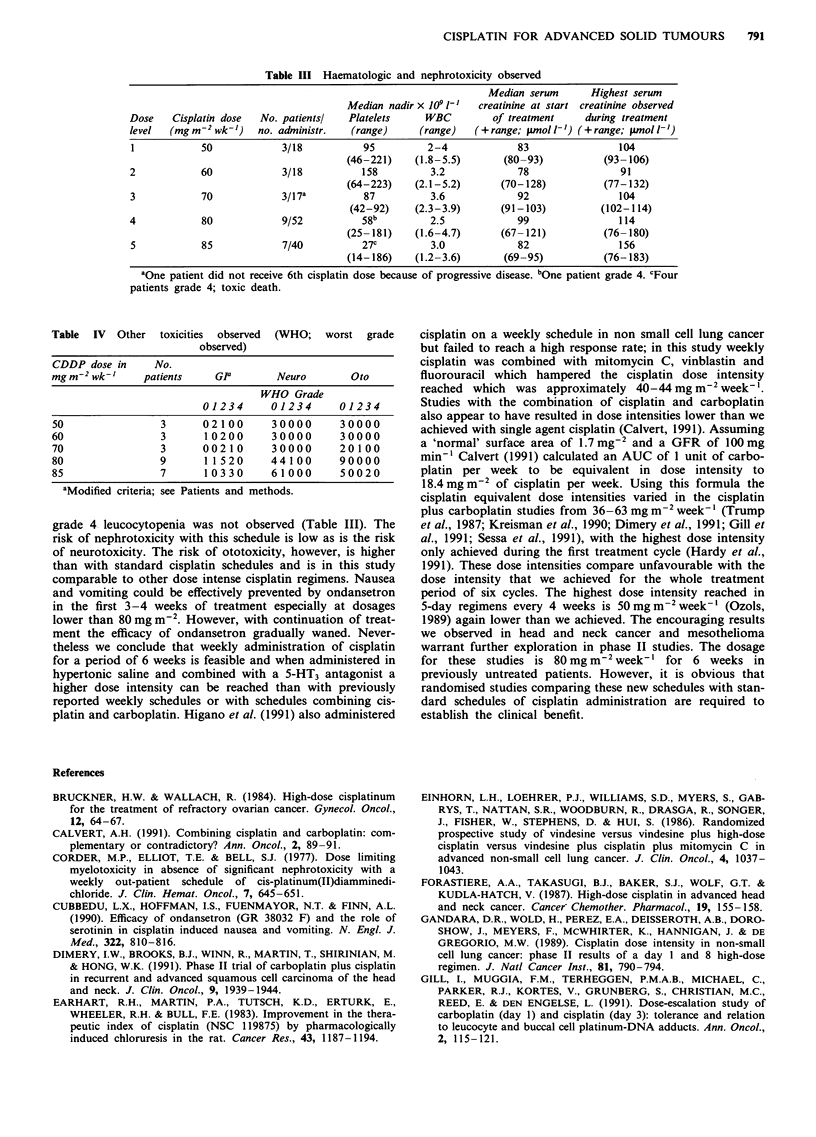
Twenty-five patients with advanced solid tumours were entered in a phase I/II study of six, weekly cycles of cisplatin. Nineteen patients were chemonaive and six were previously treated. The starting dose was 50 mg m-2 week-1. This dose could be escalated without major toxicity to 70 mg m-2 week-1. At a dose of 80 mg m-2 myelosuppression grade 3 occurred as well as grade 1 nephro- and neurotoxicity. The maximum tolerated dose was 85 mg m-2 with dose limiting thrombocytopenia. Hypertonic saline was effective in preventing nephrotoxicity. Ondansetron was a very effective antiemetic in the first weeks of treatment but its efficacy waned later on. Responses were observed in head and neck cancer, melanoma and mesothelioma. At the dose level of 80 mg m-2 the optimal dose intensity was reached. This schedule will be tested further in phase II studies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruckner H. W., Wallach R., Cohen C. J., Deppe G., Kabakow B., Ratner L., Holland J. F. High-dose platinum for the treatment of refractory ovarian cancer. Gynecol Oncol. 1981 Aug;12(1):64–67. doi: 10.1016/0090-8258(81)90095-0. [DOI] [PubMed] [Google Scholar]
- Calvert A. H. Combining cisplatin and carboplatin complementary or contradictory? Ann Oncol. 1991 Feb;2(2):89–91. doi: 10.1093/oxfordjournals.annonc.a057889. [DOI] [PubMed] [Google Scholar]
- Cubeddu L. X., Hoffmann I. S., Fuenmayor N. T., Finn A. L. Efficacy of ondansetron (GR 38032F) and the role of serotonin in cisplatin-induced nausea and vomiting. N Engl J Med. 1990 Mar 22;322(12):810–816. doi: 10.1056/NEJM199003223221204. [DOI] [PubMed] [Google Scholar]
- De Mulder P. H., Seynaeve C., Vermorken J. B., van Liessum P. A., Mols-Jevdevic S., Allman E. L., Beranek P., Verweij J. Ondansetron compared with high-dose metoclopramide in prophylaxis of acute and delayed cisplatin-induced nausea and vomiting. A multicenter, randomized, double-blind, crossover study. Ann Intern Med. 1990 Dec 1;113(11):834–840. doi: 10.7326/0003-4819-113-11-834. [DOI] [PubMed] [Google Scholar]
- Dimery I. W., Brooks B. J., Winn R., Martin T., Shirinian M., Hong W. K. Phase II trial of carboplatin plus cisplatin in recurrent and advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 1991 Nov;9(11):1939–1944. doi: 10.1200/JCO.1991.9.11.1939. [DOI] [PubMed] [Google Scholar]
- Earhart R. H., Martin P. A., Tutsch K. D., Ertürk E., Wheeler R. H., Bull F. E. Improvement in the therapeutic index of cisplatin (NSC 119875) by pharmacologically induced chloruresis in the rat. Cancer Res. 1983 Mar;43(3):1187–1194. [PubMed] [Google Scholar]
- Einhorn L. H., Loehrer P. J., Williams S. D., Meyers S., Gabrys T., Nattan S. R., Woodburn R., Drasga R., Songer J., Fisher W. Random prospective study of vindesine versus vindesine plus high-dose cisplatin versus vindesine plus cisplatin plus mitomycin C in advanced non-small-cell lung cancer. J Clin Oncol. 1986 Jul;4(7):1037–1043. doi: 10.1200/JCO.1986.4.7.1037. [DOI] [PubMed] [Google Scholar]
- Forastiere A. A., Takasugi B. J., Baker S. R., Wolf G. T., Kudla-Hatch V. High-dose cisplatin in advanced head and neck cancer. Cancer Chemother Pharmacol. 1987;19(2):155–158. doi: 10.1007/BF00254569. [DOI] [PubMed] [Google Scholar]
- Gandara D. R., Wold H., Perez E. A., Deisseroth A. B., Doroshow J., Meyers F., McWhirter K., Hannigan J., De Gregorio M. W. Cisplatin dose intensity in non-small cell lung cancer: phase II results of a day 1 and day 8 high-dose regimen. J Natl Cancer Inst. 1989 May 10;81(10):790–794. doi: 10.1093/jnci/81.10.790. [DOI] [PubMed] [Google Scholar]
- Gill I., Muggia F. M., Terheggen P. M., Michael C., Parker R. J., Kortes V., Grunberg S., Christian M. C., Reed E., den Engelse L. Dose-escalation study of carboplatin (day 1) and cisplatin (day 3): tolerance and relation to leukocyte and buccal cell platinum--DNA adducts. Ann Oncol. 1991 Feb;2(2):115–121. doi: 10.1093/oxfordjournals.annonc.a057872. [DOI] [PubMed] [Google Scholar]
- Gralla R. J., Casper E. S., Kelsen D. P., Braun D. W., Jr, Dukeman M. E., Martini N., Young C. W., Golbey R. B. Cisplatin and vindesine combination chemotherapy for advanced carcinoma of the lung: A randomized trial investigating two dosage schedules. Ann Intern Med. 1981 Oct;95(4):414–420. doi: 10.7326/0003-4819-95-4-414. [DOI] [PubMed] [Google Scholar]
- Hardy J. R., Wiltshaw E., Blake P. R., Harper P., Slevin M., Perren T. J., Tan S. Cisplatin and carboplatin in combination for the treatment of stage IV ovarian carcinoma. Ann Oncol. 1991 Feb;2(2):131–136. doi: 10.1093/oxfordjournals.annonc.a057876. [DOI] [PubMed] [Google Scholar]
- Higano C. S., Crowley J., Livingston R. B., Goodwin J. W., Barlogie B., Stuckey W. J. A weekly cisplatin-based induction regimen for extensive non-small cell lung cancer. A Southwest Oncology Group study. Cancer. 1991 May 15;67(10):2439–2442. doi: 10.1002/1097-0142(19910515)67:10<2439::aid-cncr2820671007>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Horwich A., Brada M., Nicholls J., Jay G., Hendry W. F., Dearnaley D., Peckham M. J. Intensive induction chemotherapy for poor risk non-seminomatous germ cell tumours. Eur J Cancer Clin Oncol. 1989 Feb;25(2):177–184. doi: 10.1016/0277-5379(89)90005-9. [DOI] [PubMed] [Google Scholar]
- Kaye S. B., Lewis C. R., Paul J., Duncan I. D., Gordon H. K., Kitchener H. C., Cruickshank D. J., Atkinson R. J., Soukop M., Rankin E. M. Randomised study of two doses of cisplatin with cyclophosphamide in epithelial ovarian cancer. Lancet. 1992 Aug 8;340(8815):329–333. doi: 10.1016/0140-6736(92)91404-v. [DOI] [PubMed] [Google Scholar]
- Kreisman H., Goutsou M., Modeas C., Graziano S. L., Costanza M. E., Green M. R. Cisplatin-carboplatin therapy in extensive non-small cell lung cancer: a Cancer and Leukemia Group B study. Eur J Cancer. 1990;26(10):1057–1060. doi: 10.1016/0277-5379(90)90051-t. [DOI] [PubMed] [Google Scholar]
- Levin L., Hryniuk W. M. Dose intensity analysis of chemotherapy regimens in ovarian carcinoma. J Clin Oncol. 1987 May;5(5):756–767. doi: 10.1200/JCO.1987.5.5.756. [DOI] [PubMed] [Google Scholar]
- Lewis C. R., Fossà S. D., Mead G., ten Bokkel Huinink W., Harding M. J., Mill L., Paul J., Jones W. G., Rodenburg C. J., Cantwell B. BOP/VIP--a new platinum-intensive chemotherapy regimen for poor prognosis germ cell tumours. Ann Oncol. 1991 Mar;2(3):203–211. doi: 10.1093/oxfordjournals.annonc.a057906. [DOI] [PubMed] [Google Scholar]
- Lund B., Hansen M., Hansen O. P., Hansen H. H. High-dose platinum consisting of combined carboplatin and cisplatin in previously untreated ovarian cancer patients with residual disease. J Clin Oncol. 1989 Oct;7(10):1469–1473. doi: 10.1200/JCO.1989.7.10.1469. [DOI] [PubMed] [Google Scholar]
- Marty M., Pouillart P., Scholl S., Droz J. P., Azab M., Brion N., Pujade-Lauraine E., Paule B., Paes D., Bons J. Comparison of the 5-hydroxytryptamine3 (serotonin) antagonist ondansetron (GR 38032F) with high-dose metoclopramide in the control of cisplatin-induced emesis. N Engl J Med. 1990 Mar 22;322(12):816–821. doi: 10.1056/NEJM199003223221205. [DOI] [PubMed] [Google Scholar]
- Mortimer J. E., Schulman S., MacDonald J. S., Kopecky K., Goodman G. High-dose cisplatin in disseminated melanoma: a comparison of two schedules. Cancer Chemother Pharmacol. 1990;25(5):373–376. doi: 10.1007/BF00686241. [DOI] [PubMed] [Google Scholar]
- Nichols C. R., Williams S. D., Loehrer P. J., Greco F. A., Crawford E. D., Weetlaufer J., Miller M. E., Bartolucci A., Schacter L., Einhorn L. H. Randomized study of cisplatin dose intensity in poor-risk germ cell tumors: a Southeastern Cancer Study Group and Southwest Oncology Group protocol. J Clin Oncol. 1991 Jul;9(7):1163–1172. doi: 10.1200/JCO.1991.9.7.1163. [DOI] [PubMed] [Google Scholar]
- Ozols R. F. Cisplatin dose intensity. Semin Oncol. 1989 Aug;16(4 Suppl 6):22–30. [PubMed] [Google Scholar]
- Ozols R. F., Ihde D. C., Linehan W. M., Jacob J., Ostchega Y., Young R. C. A randomized trial of standard chemotherapy v a high-dose chemotherapy regimen in the treatment of poor prognosis nonseminomatous germ-cell tumors. J Clin Oncol. 1988 Jun;6(6):1031–1040. doi: 10.1200/JCO.1988.6.6.1031. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Ostchega Y., Myers C. E., Young R. C. High-dose cisplatin in hypertonic saline in refractory ovarian cancer. J Clin Oncol. 1985 Sep;3(9):1246–1250. doi: 10.1200/JCO.1985.3.9.1246. [DOI] [PubMed] [Google Scholar]
- Pillay C. V., Green-Thompson R., Brock-Utne J. G. Efficacy of the anticancer agent cisplatin in the treatment of human cervical squamous carcinoma xenografted in nude mice. Chemotherapy. 1986;32(4):356–363. doi: 10.1159/000238435. [DOI] [PubMed] [Google Scholar]
- Randolph V. L., Wittes R. E. Weekly administration of cis-diamminedichloroplatinum (II) without hydration or osmotic diuresis. Eur J Cancer. 1978 Jul;14(7):753–756. doi: 10.1016/0014-2964(78)90004-x. [DOI] [PubMed] [Google Scholar]
- Samson M. K., Rivkin S. E., Jones S. E., Costanzi J. J., LoBuglio A. F., Stephens R. L., Gehan E. A., Cummings G. D. Dose-response and dose-survival advantage for high versus low-dose cisplatin combined with vinblastine and bleomycin in disseminated testicular cancer. A Southwest Oncology Group study. Cancer. 1984 Mar 1;53(5):1029–1035. doi: 10.1002/1097-0142(19840301)53:5<1029::aid-cncr2820530503>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sessa C., Goldhirsch A., Martinelli G., Alerci M., Imburgia L., Cavalli F. Phase I study of the combination of monthly carboplatin and weekly cisplatin. Ann Oncol. 1991 Feb;2(2):123–129. doi: 10.1093/oxfordjournals.annonc.a057874. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Newlands E. S., Rustin G. J., Begent R. H., Crawford S. M., Bagshawe K. D., Carruthers L. A phase I/II study of the 5-HT3 antagonist GR38032F in the anti-emetic prophylaxis of patients receiving high-dose cisplatin chemotherapy. Cancer Chemother Pharmacol. 1990;25(4):291–294. doi: 10.1007/BF00684888. [DOI] [PubMed] [Google Scholar]
- Trump D. L., Grem J. L., Tutsch K. D., Willson J. K., Simon K. J., Alberti D., Storer B., Tormey D. C. Platinum analogue combination chemotherapy: cisplatin and carboplatin--a phase I trial with pharmacokinetic assessment of the effect of cisplatin administration on carboplatin excretion. J Clin Oncol. 1987 Aug;5(8):1281–1289. doi: 10.1200/JCO.1987.5.8.1281. [DOI] [PubMed] [Google Scholar]



