Abstract
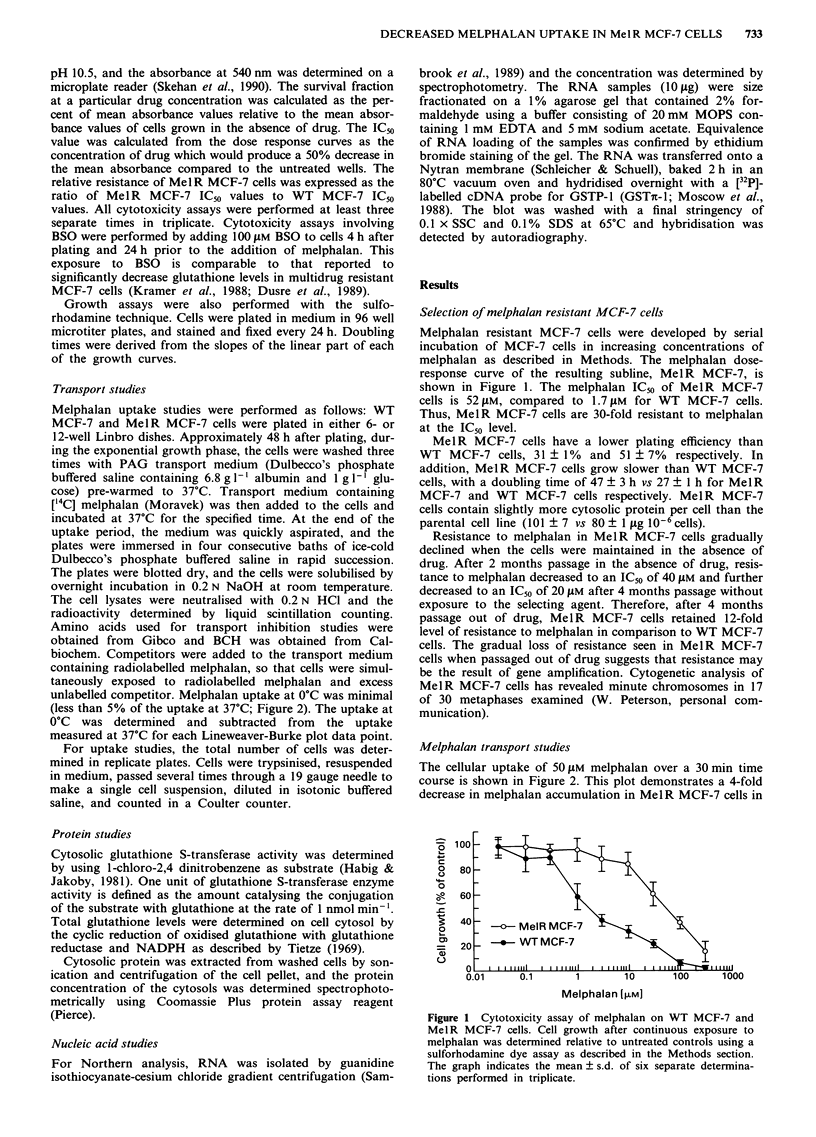
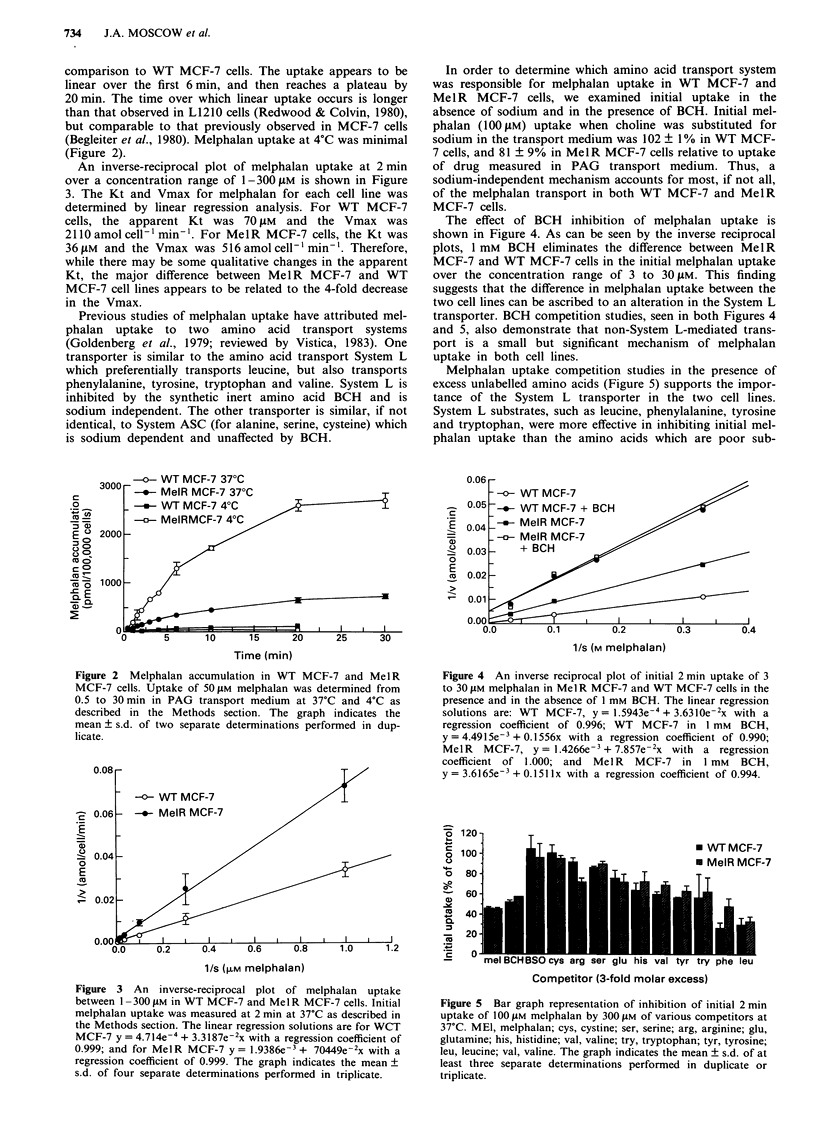
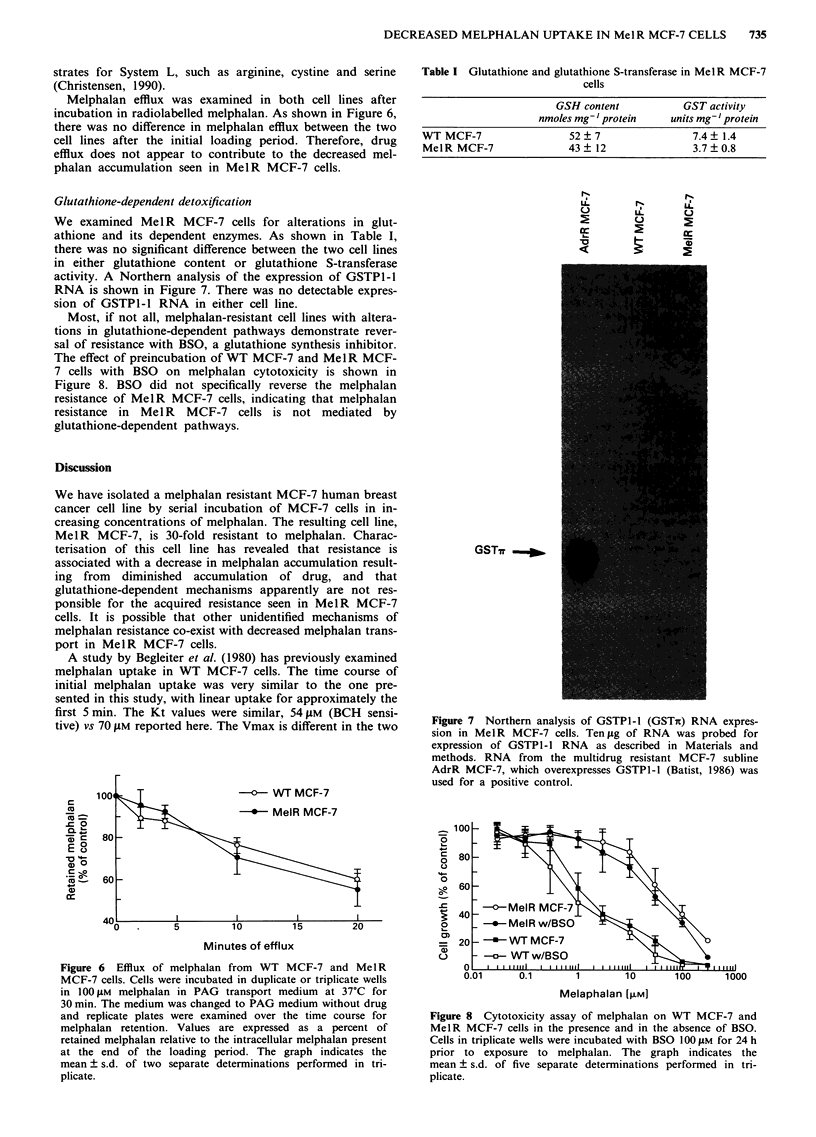
An in vitro model of acquired melphalan resistance was developed by serial incubation of an MCF-7 human breast cancer cell line in increasing concentrations of melphalan. The resulting derivative cell line, Me1R MCF-7, was 30-fold resistant to melphalan. Uptake studies demonstrated decreased initial melphalan accumulation in Me1R MCF-7 cells. Inverse-reciprocal plots of initial melphalan uptake revealed a 4-fold decrease in the apparent Vmax of Me1R MCF-7 compared with WT MCF-7 (516 amol cell-1 min-1 vs 2110 amol cell-1 min-1 respectively) as well as a decrease in the apparent Kt (36 microM vs 70 microM respectively). Two amino acid transporters have previously been identified as melphalan transporters: system L, which is sodium-independent and inhibited by 2-amino-bicyclo[2,2,1]heptane-2-carboxylic acid (BCH), and system ASC which is sodium dependent and unaffected by BCH. At low concentrations of melphalan (3-30 microM), 1mM BCH competition eliminated the differences between the two cell lines, thus implicating an alteration of the system L transporter in the transport defect in the resistant cells. Me1R MCF-7 cells were also evaluated for glutathione-mediated detoxification mechanisms associated with melphalan resistance. There was no difference between Me1R MCF-7 and WT MCF-7 in glutathione content, glutathione-S-transferase activity and expression of pi class glutathione S-transferase RNA. In addition, buthionine sulfoximine did not reverse melphalan resistance in Me1R MCF-7 cells. Therefore, Me1R MCF-7 cells provide an in vitro model of transport-mediated melphalan resistance in human breast cancer cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Okine L., Le B., Najarian P., Vistica D. T. Elevation of glutathione in phenylalanine mustard-resistant murine L1210 leukemia cells. J Biol Chem. 1987 Nov 5;262(31):15048–15053. [PubMed] [Google Scholar]
- Ahmad S., Okine L., Wood R., Aljian J., Vistica D. T. gamma-Glutamyl transpeptidase (gamma-GT) and maintenance of thiol pools in tumor cells resistant to alkylating agents. J Cell Physiol. 1987 May;131(2):240–246. doi: 10.1002/jcp.1041310214. [DOI] [PubMed] [Google Scholar]
- Bailey H. H., Gipp J. J., Ripple M., Wilding G., Mulcahy R. T. Increase in gamma-glutamylcysteine synthetase activity and steady-state messenger RNA levels in melphalan-resistant DU-145 human prostate carcinoma cells expressing elevated glutathione levels. Cancer Res. 1992 Sep 15;52(18):5115–5118. [PubMed] [Google Scholar]
- Batist G., Torres-Garcia S., Demuys J. M., Greene D., Lehnert S., Rochon M., Panasci L. Enhanced DNA cross-link removal: the apparent mechanism of resistance in a clinically relevant melphalan-resistant human breast cancer cell line. Mol Pharmacol. 1989 Aug;36(2):224–230. [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Begleiter A., Froese E. K., Goldenberg G. J. A comparison of melphalan transport in human breast cancer cells and lymphocytes in vitro. Cancer Lett. 1980 Sep;10(3):243–251. doi: 10.1016/0304-3835(80)90077-4. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Grover J., Froese E., Goldenberg G. J. Membrane transport, sulfhydryl levels and DNA cross-linking in Chinese hamster ovary cell mutants sensitive and resistant to melphalan. Biochem Pharmacol. 1983 Jan 15;32(2):293–300. doi: 10.1016/0006-2952(83)90558-0. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Grover J., Goldenberg G. J. Mechanism of efflux of melphalan from L5178Y lymphoblasts in vitro. Cancer Res. 1982 Mar;42(3):987–991. [PubMed] [Google Scholar]
- Bellamy W. T., Dalton W. S., Gleason M. C., Grogan T. M., Trent J. M. Development and characterization of a melphalan-resistant human multiple myeloma cell line. Cancer Res. 1991 Feb 1;51(3):995–1002. [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990 Jan;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Dantzig A. H., Fairgrieve M., Slayman C. W., Adelberg E. A. Isolation and characterization of a CHO amino acid transport mutant resistant to melphalan (L-phenylalanine mustard). Somat Cell Mol Genet. 1984 Mar;10(2):113–121. doi: 10.1007/BF01534900. [DOI] [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C. Conversion of melphalan to 4-(glutathionyl)phenylalanine. A novel mechanism for conjugation by glutathione-S-transferases. Drug Metab Dispos. 1987 Mar-Apr;15(2):195–199. [PubMed] [Google Scholar]
- Dusre L., Mimnaugh E. G., Myers C. E., Sinha B. K. Potentiation of doxorubicin cytotoxicity by buthionine sulfoximine in multidrug-resistant human breast tumor cells. Cancer Res. 1989 Feb 1;49(3):511–515. [PubMed] [Google Scholar]
- Elliott E. M., Ling V. Selection and characterization of Chinese hamster ovary cell mutants resistant to melphalan (L-phenylalanine mustard). Cancer Res. 1981 Feb;41(2):393–400. [PubMed] [Google Scholar]
- Friedman H. S., Colvin O. M., Griffith O. W., Lippitz B., Elion G. B., Schold S. C., Jr, Hilton J., Bigner D. D. Increased melphalan activity in intracranial human medulloblastoma and glioma xenografts following buthionine sulfoximine-mediated glutathione depletion. J Natl Cancer Inst. 1989 Apr 5;81(7):524–527. doi: 10.1093/jnci/81.7.524. [DOI] [PubMed] [Google Scholar]
- Friedman H. S., Skapek S. X., Colvin O. M., Elion G. B., Blum M. R., Savina P. M., Hilton J., Schold S. C., Jr, Kurtzberg J., Bigner D. D. Melphalan transport, glutathione levels, and glutathione-S-transferase activity in human medulloblastoma. Cancer Res. 1988 Oct 1;48(19):5397–5402. [PubMed] [Google Scholar]
- Goldenberg G. J., Lam H. Y., Begleiter A. Active carrier-mediated transport of melphalan by two separate amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J Biol Chem. 1979 Feb 25;254(4):1057–1064. [PubMed] [Google Scholar]
- Green J. A., Vistica D. T., Young R. C., Hamilton T. C., Rogan A. M., Ozols R. F. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res. 1984 Nov;44(11):5427–5431. [PubMed] [Google Scholar]
- Groothuis D. R., Lippitz B. E., Fekete I., Schlageter K. E., Molnar P., Colvin O. M., Roe C. R., Bigner D. D., Friedman H. S. The effect of an amino acid-lowering diet on the rate of melphalan entry into brain and xenotransplanted glioma. Cancer Res. 1992 Oct 15;52(20):5590–5596. [PubMed] [Google Scholar]
- Gupta V., Singh S. V., Ahmad H., Medh R. D., Awasthi Y. C. Glutathione and glutathione S-transferases in a human plasma cell line resistant to melphalan. Biochem Pharmacol. 1989 Jun 15;38(12):1993–2000. doi: 10.1016/0006-2952(89)90499-1. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Greene K., Ahmad S., Vistica D. T. Chemosensitization of L-phenylalanine mustard by the thiol-modulating agent buthionine sulfoximine. Cancer Res. 1987 Mar 15;47(6):1593–1597. [PubMed] [Google Scholar]
- Kramer R. A., Zakher J., Kim G. Role of the glutathione redox cycle in acquired and de novo multidrug resistance. Science. 1988 Aug 5;241(4866):694–697. doi: 10.1126/science.3399900. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B. R., Townsend A. J., Tu C. P., Cowan K. H., Goldsmith M. E. Antineoplastic drug sensitivity of human MCF-7 breast cancer cells stably transfected with a human alpha class glutathione S-transferase gene. Cancer Res. 1991 Jan 15;51(2):587–594. [PubMed] [Google Scholar]
- Moscow J. A., Townsend A. J., Cowan K. H. Elevation of pi class glutathione S-transferase activity in human breast cancer cells by transfection of the GST pi gene and its effect on sensitivity to toxins. Mol Pharmacol. 1989 Jul;36(1):22–28. [PubMed] [Google Scholar]
- Moscow J. A., Townsend A. J., Goldsmith M. E., Whang-Peng J., Vickers P. J., Poisson R., Legault-Poisson S., Myers C. E., Cowan K. H. Isolation of the human anionic glutathione S-transferase cDNA and the relation of its gene expression to estrogen-receptor content in primary breast cancer. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6518–6522. doi: 10.1073/pnas.85.17.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K., Saijo N., Tsuchida S., Sakai M., Tsunokawa Y., Yokota J., Muramatsu M., Sato K., Terada M., Tew K. D. Glutathione-S-transferase pi as a determinant of drug resistance in transfectant cell lines. J Biol Chem. 1990 Mar 15;265(8):4296–4301. [PubMed] [Google Scholar]
- Puchalski R. B., Fahl W. E. Expression of recombinant glutathione S-transferase pi, Ya, or Yb1 confers resistance to alkylating agents. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2443–2447. doi: 10.1073/pnas.87.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood W. R., Colvin M. Transport of melphalan by sensitive and resistant L1210 cells. Cancer Res. 1980 Apr;40(4):1144–1149. [PubMed] [Google Scholar]
- Rosenberg M. C., Colvin O. M., Griffith O. W., Bigner S. H., Elion G. B., Horton J. K., Lilley E., Bigner D. D., Friedman H. S. Establishment of a melphalan-resistant rhabdomyosarcoma xenograft with cross-resistance to vincristine and enhanced sensitivity following buthionine sulfoximine-mediated glutathione depletion. Cancer Res. 1989 Dec 15;49(24 Pt 1):6917–6922. [PubMed] [Google Scholar]
- Schecter R. L., Woo A., Duong M., Batist G. In vivo and in vitro mechanisms of drug resistance in a rat mammary carcinoma model. Cancer Res. 1991 Mar 1;51(5):1434–1442. [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Smith A. C., Liao J. T., Page J. G., Wientjes M. G., Grieshaber C. K. Pharmacokinetics of buthionine sulfoximine (NSC 326231) and its effect on melphalan-induced toxicity in mice. Cancer Res. 1989 Oct 1;49(19):5385–5391. [PubMed] [Google Scholar]
- Tabibi S. E., Cradock J. C. Stability of melphalan in infusion fluids. Am J Hosp Pharm. 1984 Jul;41(7):1380–1382. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Vistica D. T. Cellular pharmacokinetics of the phenylalanine mustards. Pharmacol Ther. 1983;22(3):379–406. doi: 10.1016/0163-7258(83)90009-8. [DOI] [PubMed] [Google Scholar]
- Vistica D. T., Schuette B. P. Carrier mechanism and specificity accounting for the increase in intracellular melphalan by the basic amino acids. Mol Pharmacol. 1981 Jan;19(1):92–96. [PubMed] [Google Scholar]




