Abstract
Sphingosine 1-phosphate (S1P) is a platelet-derived sphingolipid that binds to S1P1 (EDG-1) receptors and activates the endothelial isoform of NO synthase (eNOS). S1P and the polypeptide growth factor vascular endothelial growth factor (VEGF) act independently to modulate angiogenesis and activate eNOS. In these studies, we explored the cross-talk between S1P and VEGF signaling pathways. When cultured bovine aortic endothelial cells were treated with VEGF (10 ng/ml), the expression of S1P1 protein and mRNA increased by ≈4-fold. S1P1 up-regulation by VEGF was seen within 30 min of VEGF addition and reached a maximum after 1.5 h. By contrast, expression of neither bradykinin B2 receptors nor the scaffolding protein caveolin-1 was altered by VEGF treatment. The EC50 for VEGF-promoted induction of S1P1 expression was ≈2 ng/ml, within its physiological concentration range. S1P1 induction by VEGF was attenuated by the tyrosine kinase inhibitor genistein and by the PKC inhibitor calphostin C. Preincubation of bovine aortic endothelial cells with VEGF (10 ng/ml for 90 min) markedly enhanced subsequent S1P-dependent eNOS activation. VEGF pretreatment of cultured endothelial cells also markedly potentiated S1P-promoted eNOS phosphorylation at Ser-1179, as well as S1P-mediated activation of kinase Akt. In isolated rat arteries, VEGF pretreatment markedly potentiated S1P-mediated vasorelaxation and eNOS Ser-1179 phosphorylation. Taken together, these data indicate that VEGF specifically induces expression of S1P1 receptors, associated with enhanced intracellular signaling responses to S1P and the potentiation of S1P-mediated vasorelaxation. We suggest that VEGF acts to sensitize the vascular endothelium to the effects of lipid mediators by promoting the induction of S1P1 receptors, representing a potentially important point of cross-talk between receptor-regulated eNOS signaling pathways in the vasculature.
The endothelial isoform of nitric oxide synthase (eNOS) is a key signaling protein in vascular endothelial cells and modulates a wide array of essential vascular functions, including regulation of blood pressure, inhibition of platelet aggregation, and angiogenic responses (reviewed in ref. 1). eNOS activity is complexly modulated by a wide variety of physiological and pathophysiological stimuli, including hormones such as bradykinin (2), growth factors such as vascular endothelial growth factor (VEGF) (3), and mechanical stimuli (4). The latest additions to this list of eNOS-activating stimulators include sphingosine 1-phosphate (S1P) (5–7). S1P is a platelet-derived sphingolipid mediator that modulates many important functions of vascular endothelial cells, including angiogenic morphogenesis, inhibition of apoptosis, chemotactic responses, and proliferation, among others (reviewed in ref. 8). Most, if not all, of these S1P actions are mediated by binding to and activating a novel family of G protein-coupled receptors termed EDG receptors, recently renamed S1P receptors (9). We have previously explored the mechanisms whereby S1P activates eNOS in endothelial cells. eNOS activation by S1P is mediated by the activation of S1P receptors that are coupled to pertussis toxin-sensitive G proteins, leading to the elevation of intracellular calcium levels (10, 11) and the activation of the β-isoform of phosphoinositide 3-kinase (PI3-Kβ), which then activates the protein kinase Akt, which in turn phosphorylates eNOS at Ser-1179 (5–7). Other recent reports have explored the physiological consequences of eNOS activation by S1P and have shown that eNOS activation by S1P may mediate several important endothelial pathways, including inhibition of apoptosis (12, 13), vasorelaxation (14), and angiogenesis (15).
VEGF has been extensively characterized as an angiogenic polypeptide growth factor and is known to modulate numerous endothelial functions that are mediated by its receptor, KDR (reviewed in ref. 16). Like S1P, VEGF activates eNOS (3, 7); both S1P and VEGF independently (and, possibly, synergistically) stimulate angiogenesis (15, 17, 18). Thus, S1P and VEGF elicit similar receptor-mediated biological responses in vascular endothelial cells, yet the molecular basis whereby these two receptor pathways interact with each other remains less well understood. The principal S1P receptor isoform expressed in vascular endothelial cells is the S1P1 receptor subtype (11, 17, 19). Interestingly, the S1P1 receptor was originally identified and isolated as an immediate early gene product that is abundantly induced after activation of vascular endothelial cells (20), suggesting that endothelial S1P1 receptor expression may be subject to (patho)physiological regulation.
These observations led us to hypothesize that VEGF might alter the expression of S1P1 receptors in vascular endothelial cells. In the present studies, we demonstrate that VEGF treatment of vascular endothelial cells markedly up-regulates S1P1 expression and enhances S1P-mediated signaling pathways leading to eNOS activation. These experiments may identify a novel mode of cross-talk between G protein-coupled receptor- and growth factor receptor-mediated signaling pathways in vascular endothelial cells.
Experimental Procedures
Materials. FBS was from HyClone. All other cell culture reagents and media were from Life Technologies (Rockville, MD). S1P was from Biomol (Plymouth Meeting, PA). Genistein, wortmannin, PD98059, calphostin C, and phorbol 12-myristate 13-acetate (PMA), as well as various polypeptide growth factors including VEGF, were from Calbiochem. Anti-S1P1 antibody, raised against N-terminal fragment peptide of rat EDG-1 (S1P1) (21), was a gift from R. A. Sabbadini (San Diego State University, San Diego). Anti-EDG-3 (S1P3) and EDG-5 (S1P2) polyclonal antibodies were from ExAlpha (Watertown, MA). Anti-phospho-eNOS antibody (phosphoserine-1179 in bovine eNOS sequence), anti-phospho-Akt antibody (Ser-473), anti-Akt antibody, and anti-phospho-ERK1/2 antibody (Thr-202/Tyr-204) were from Cell Signaling Technology (Beverly, MA). Anti-eNOS and anti-bradykinin B2 receptor monoclonal antibodies and anti-caveolin polyclonal antibody were from Transduction Laboratories (Lexington, KY). Anti-KDR monoclonal antibody (A-3) was from Santa Cruz Biotechnology. SuperBlock reagents, SuperSignal substrates for chemiluminescence detection, and secondary antibodies conjugated with horseradish peroxidase were from Pierce. RNeasy minicolumns were from Qiagen (Valencia, CA). The cDNA plasmid encoding human full-length S1P1 (EDG-1) tagged with FLAG peptide was provided by T. Hla (University of Connecticut) (22). Human GAPDH cDNA was from Clontech. Hybond-N+ nylon membranes, Mega-Prime random priming kit, ProbeQuant G-50 micro columns, [32P]CTP, and l-[3H]arginine were from Amersham Pharmacia Biotech. Protein determinations were made with the Bio-Rad Protein Assay Kit. All other materials were from Sigma.
Cell Culture and Drug Treatments. Bovine aortic endothelial cells (BAEC) were obtained from Cell Systems (Kirkland, WA), maintained in culture as described (6), and used for experiments between passages 5 and 7. Cells had been serum-starved overnight before being used for experiments to exclude the effects of residual S1P in FBS (6, 11). S1P was dissolved into 0.4% (wt/vol) fatty-acid-free BSA as described (23). All other drug treatments were performed exactly as described (6, 24).
Immunoblot Analyses in Cultured Cells. Cells were extensively washed with ice-cold PBS and then directly harvested in cell lysis buffer containing Tris·HCl (60 mM, pH 6.8), DTT (100 mM), glycerol (5%, wt/vol), and SDS (1.7%, wt/vol). For immunoblots using the EDG (S1P) receptor antibodies, the harvested cells were lysed by multiple passes through a 21-gauge needle followed by sonication on ice [Branson Sonifier 450, 3 × 10 s (25)]. This method for processing cell lysates for SDS/PAGE/immunoblot analyses of S1P receptors had been previously optimized (26). Cell lysates were resolved by SDS/PAGE on 9% gels and transferred to nitrocellulose membranes, which were blocked with SuperBlock solution for 2 h at room temperature and then incubated with an anti-EDG antibody, followed by an incubation with horseradish peroxidase-conjugated secondary antibody in Tris-buffered saline containing 5% milk. The immunoreactive signals were visualized by using SuperSignal West Femto Maximum Sensitivity Substrate and exposed by using Kodak X-Omat film. All other immunoblot analyses were performed by using standard protocols as described in detail elsewhere (6, 24) by using SuperSignal West Pico substrate.
Northern Blot Analyses. Northern blot analyses for S1P1 transcripts in BAEC were performed essentially as described (20). Briefly, total RNA was isolated from BAEC culture by using an RNeasy minicolumn kit (Qiagen) following the manufacturer's protocol. Typically, ≈30–50 μg of total RNA was obtained from cells on a 100-mm culture dish. Equal quantities of RNA were resolved on a 1% agarose gel containing 2.2 M formaldehyde, capillary-blotted onto a nylon membrane, and then UV-cross-linked. Full-length cDNA inserts corresponding to full-length human S1P1 (EDG-1) (22) or GAPDH (Clontech) were labeled to a high specific activity (<106 cpm/μg DNA) by using a random primer labeling kit (Amersham Pharmacia Biotech). Membranes were then hybridized, extensively washed as described (20), and subjected to autoradiography. Typically, the membranes were exposed at –80°C overnight.
Quantitation of Intracellular NO Generation. eNOS enzyme activity was quantified as the intracellular formation of l-[3H]citrulline from l-[3H]arginine, as described (5–7).
Analyses in Intact Blood Vessels. Vascular tone was studied by using video microscopy in isolated pressurized superfused rat mesenteric arteries, as described in detail (14). Isolated arterioles were incubated at 60 mmHg (1 mmHg = 133 Pa) pressure for 90 min with VEGF (20 ng/ml in 0.1% BSA) or vehicle in a physiological chamber (Living Systems Instruments, Burlington, VT) at 37°C, and then constricted with 5 μM norepinephrine before treatments for 5 min with S1P (100 nM) or BK (1 μM). Vessel diameter measurements were quantitated and analyzed by using ionoptix software (Ionoptix, Milton, MA); vessel relaxation is normalized as the fractional reversal of norepinephrine-induced vessel contraction. High-sensitivity immunoblot analyses in intact blood vessels were performed as described (14).
Other Methods. All experiments were performed at least three times. Mean values for individual experiments are expressed as mean ± SEM. Statistical differences were analyzed by ANOVA followed by Scheffé's F test using stat view ii (Abacus Concepts, Berkeley, CA). A P value <0.05 was considered statistically significant.
Results
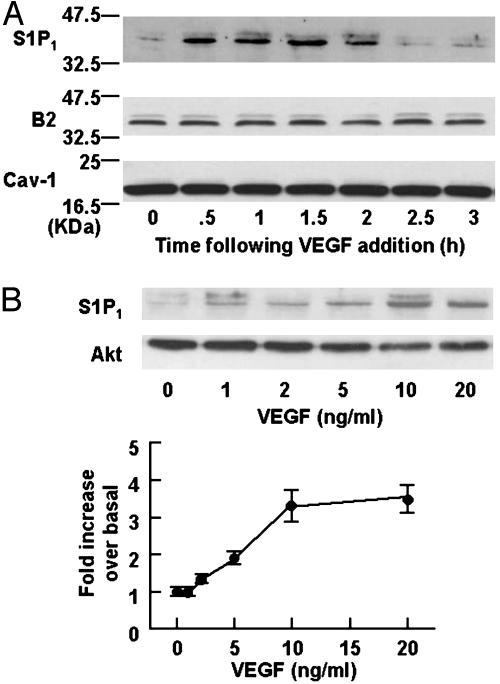
We first studied the effects of VEGF on the expression levels of S1P1 receptor protein in BAEC. As shown in Fig. 1A, we detected a significant immunoreactive signal of S1P1 receptors in the resting BAEC. However, when cells were treated with VEGF (10 ng/ml), the expression of S1P1 receptor protein markedly increased within 30 min of drug addition (Fig. 1 A). The converse experiment, in which BAEC are treated for varying times with S1P (100 nM) and then analyzed for expression of the VEGF receptor KDR, reveals no effect of S1P on KDR protein expression levels (data not shown). The VEGF-induced increase in S1P1 receptor protein is maximal at 1.5 h and returns to basal levels by 3 h (Fig. 1 A). We also analyzed these same BAEC lysates in immunoblots that were probed with antibodies directed against several other signaling proteins. VEGF (10 ng/ml for 90 min) did not increase the expression levels of S1P2 (EDG-5) or S1P3 (EDG-3) receptor subtypes either (data not shown). As shown in Fig. 1, VEGF does not alter the abundance of several other signaling proteins; levels of protein kinase Akt, B2 bradykinin receptors, eNOS, and scaffolding/regulatory protein caveolin-1 are all unchanged by VEGF treatment.
Fig. 1.
Effects of VEGF on expression of endothelial signaling proteins. Shown are the results of a protein immunoblot assay analyzed in cell lysates derived from BAEC treated with VEGF. (A) The results of time course experiments. BAEC were treated with VEGF (10 ng/ml) for the times indicated, and equal quantities of cellular protein (20 μg per lane) were resolved by SDS/PAGE, transferred to a nitrocellulose membrane, and subjected to immunoblot analyses probed with antibodies directed against S1P1, bradykinin B2 receptors (B2), or caveolin-1 (Cav-1), as indicated. The experiment shown is representative of four independent experiments that produced equivalent results. (B Upper) The results of dose–response experiments. BAEC were treated with VEGF for 1.5 h at the indicated concentrations. Equal quantities of cellular protein (20 μg per lane) were analyzed in immunoblots probed with an antibody specific to S1P1 or to kinase Akt, as indicated. The data shown are representative of four independent experiments that yielded equivalent results. (Lower) The results of densitometric analyses from pooled data, plotting the fold increase of the degree of expression levels of S1P1 receptors at the VEGF concentration indicated, relative to the signals obtained in the absence of VEGF. Each data point represents the mean ± SEM derived from four independent experiments.
A dose response for VEGF-mediated S1P1 receptor induction is shown in Fig. 1B. In these dose–response experiments, BAEC are treated with various concentrations of VEGF for 90 min, and the lysates derived from these cells are analyzed in immunoblots probed with the S1P1 receptor antibody. As can be seen in Fig. 1B, the EC50 for VEGF-mediated S1P1 induction is ≈5ng/ml, a value within the physiological range for many other endothelial responses of this growth factor (16). Other polypeptide growth factors, including basic fibroblast growth factor and hepatocyte growth factor (10 ng/ml), as well as S1P (100 nM), were also without effect for the expression levels of S1P1 protein (data not shown).
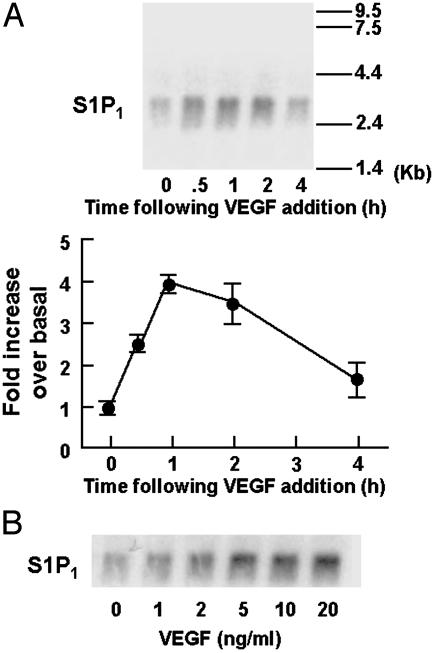
We next performed Northern blot analyses of RNA isolated from VEGF-treated endothelial cells, using the S1P1 cDNA as probe (22). As shown in Fig. 2A, the abundance of S1P1 transcripts increases within 30 min of VEGF addition to BAEC. The VEGF-induced increase in S1P1 mRNA persists for ≈2 h, and returns to basal levels within 4 h of VEGF treatment. A dose–response experiment exploring the VEGF-induced increase in S1P1 mRNA is shown in Fig. 2B and reveals that the VEGF response has an EC50 of ≈2 ng/ml, a value similar to that seen for the VEGF-induced increase in S1P1 protein abundance (Fig. 1B).
Fig. 2.
Effects of VEGF on S1P1 transcript abundance. Shown are the results of Northern blots probed with S1P1 cDNA, analyzed in BAEC treated with VEGF. (A Upper) The results of time course experiments. BAEC were treated with VEGF (10 ng/ml) for the times indicated. Equal quantities of total RNA (10 μg per lane) were subjected to Northern blot analyses and probed for S1P1 transcript abundance, as described in detail in Experimental Procedures. The same membrane was also probed for GAPDH to confirm equal loading (data not shown). (Lower) The results of densitometric analyses from pooled data, plotting the fold increase of the degree of expression levels of S1P1 transcripts at the VEGF concentration indicated, relative to the signals obtained in the absence of VEGF. The experiment shown is the representative of four independent experiments. (B) The results of dose–response experiments. BAEC were treated with varying concentrations of VEGF for 60 min and then processed for Northern blot analyses and probed for S1P1 expression exactly as described above. The same membrane was also reprobed for GAPDH to confirm equal loading (data not shown). The experiment shown is representative of four independent experiments.
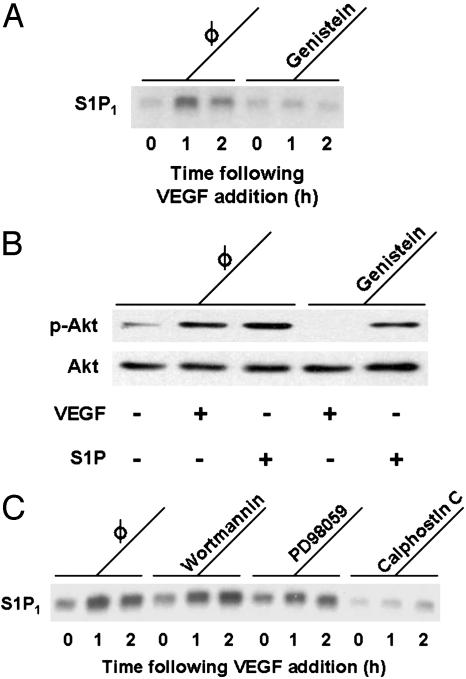
We next sought to identify the protein kinase pathway(s) involved in the VEGF-induced increase in S1P1 abundance. Previous reports have implicated several protein kinases in VEGF-induced gene regulation in vascular endothelial cells, involving tyrosine kinase as well as serine/threonine kinase pathways (16). When BAEC are treated with the protein tyrosine kinase inhibitor genistein (100 μM for 30 min), the subsequent VEGF-induced increase in S1P1 abundance is completely abrogated (Fig. 3A). Under these same conditions, genistein blocks the VEGF-induced increase in phosphorylation of the kinase Akt at serine 473 (Fig. 3B). By contrast, S1P-induced Akt phosphorylation is not blocked by genistein (Fig. 3B). Like S1P, VEGF has been implicated in multiple distinct serine/threonine kinase pathways, including the PI3-K/Akt (27), mitogen-activated protein (MAP) kinase (28), and PKC pathways (29). As shown in Fig. 3C, neither the PI3-K/Akt pathway inhibitor wortmannin nor the MAP kinase pathway inhibitor PD98059 blocks the VEGF-induced increase in S1P1 transcript abundance. By contrast, the PKC inhibitor calphostin C completely blocks the VEGF-induced increase in S1P1 mRNA (Fig. 3C). PMA, which stimulates PKC and has been previously used to induce and isolate S1P1 transcripts in endothelial cells (20), markedly augmented S1P1 expression in BAEC as well (10 ng/ml of PMA for 60 min, data not shown).
Fig. 3.
Effects of protein kinase inhibitors on VEGF-mediated responses. (A) The effects of genistein, a protein tyrosine kinase inhibitor, on the VEGF-induced increase in S1P1 mRNA abundance. BAEC were treated with genistein (100μM for 30 min) or vehicle, then incubated with VEGF (10 ng/ml) for the times indicated. Equal quantities of total RNA (10 μg per lane) derived from these cells were resolved on denaturing agarose gels, transferred to a nylon membrane, and probed with cDNA encoding S1P1. Equal loading of samples was confirmed by reprobing the membrane for GAPDH (data not shown). The data shown are representative of four independent experiments, which yielded identical results. (B) The results of immunoblot analyses of BAEC preincubated with genistein (100 μM, 30 min) or vehicle, then treated with VEGF (10 ng/ml, 5 min) or S1P (100 nM, 5 min). Equal quantities of total protein (25 μg per lane) were resolved by SDS/PAGE and analyzed in a protein immunoblot that was probed with antibodies directed against phospho-Akt (p-Akt); the same immunoblot filter was then stripped and reprobed for total Akt, as indicated. This figure is representative of three independent experiments that yielded equivalent results. (C) The effects of inhibitors of serine/threonine protein kinase pathways on the induction of S1P1 transcripts by VEGF. Before VEGF treatment (10 ng/ml for the times indicated), BAEC were treated for 30 min either with vehicle or with wortmannin (1 μM), an inhibitor of the PI3-K/Akt pathway; PD98059 (20 μM), an inhibitor of the MAP kinase pathway; or calphostin C (400 nM), an inhibitor of the PKC pathway. Equal quantities of RNA (10 μg per lane) were analyzed in Northern blots probed with S1P1 cDNA. Equal loading of samples was confirmed by reprobing the membrane for GAPDH (data not shown). The data shown are representative of four independent experiments that produced identical results.
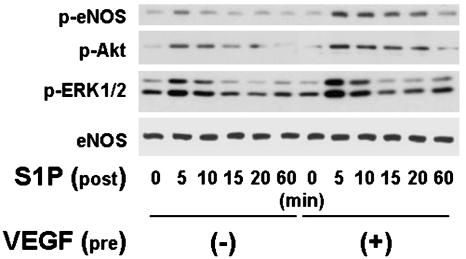
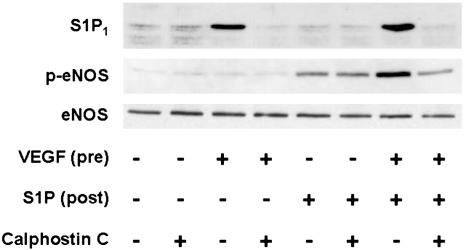
The functional consequences of the induction of S1P1 receptors in VEGF-treated BAEC were explored in a series of experiments. S1P has been previously shown to promote the phosphorylation of eNOS at Ser-1179, and also to lead to the phosphorylation of protein kinases Akt and ERK1/2 (6). We performed immunoblot analyses in lysates prepared from VEGF-pretreated BAEC that had been subsequently exposed to S1P by using as probes a series of phospho-specific antibodies. Fig. 4 shows the results of Western blot analysis using antibodies specific to phosphorylated forms of Akt, ERK1/2, and eNOS (Ser-1179) in these cells. BAEC are first incubated with VEGF (10 ng/ml for 90 min) or its vehicle, then treated with S1P (100 nM for up to 60 min). S1P induces phosphorylation of all these proteins in BAEC that had not been pretreated with VEGF (Fig. 4; see also ref. 6). However, when BAEC are first incubated with VEGF for 90 min, the subsequent S1P-induced phosphorylation of Akt, ERK1/2, and Ser-1179 eNOS are all strikingly augmented (Fig. 4). We next performed eNOS activity assays in BAEC treated under these conditions. S1P promotes eNOS activation even in BAEC that had not been pretreated with VEGF (Fig. 5; see also ref. 6). However, when BAEC are first preincubated with VEGF and then treated with S1P, the degree of eNOS activation is markedly augmented (Fig. 5). The PKC inhibitor calphostin C completely blocks the ability of VEGF to enhance S1P-mediated eNOS phosphorylation at Ser-1179, while having no effect on the S1P-elicited phosphorylation response in control BAEC not treated with VEGF (Fig. 6).
Fig. 4.
Effects of VEGF on S1P-mediated phosphorylation of Akt, ERK1/2, and eNOS. Shown are the results of immunoblots probed with phospho-specific antibodies directed against phosphorylated forms of eNOS, Akt, and ERK1/2. BAEC were incubated with VEGF (10 ng/ml for 90 min) or with vehicle, as indicated, then treated with S1P (100 nM). After addition of S1P, cells were harvested at the times indicated, and equal quantities of cell lysate (20 μg per lane) were resolved by SDS/PAGE, transferred to a nitrocellulose membrane, and probed with antibodies directed against Ser-1179 phospho-eNOS, phospho-Akt, and phospho-ERK1/2. Equal loading of samples was confirmed by reprobing the immunoblots with an antibody against (total) eNOS. Shown are representative data from an experiment that was independently repeated five times with equivalent results.
Fig. 5.
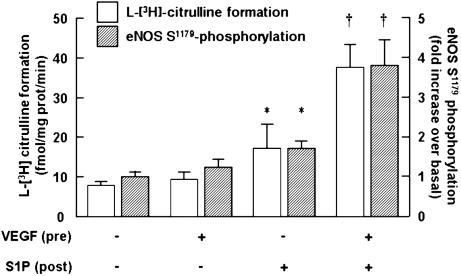
Effects of VEGF treatment on S1P-mediated eNOS activation and Ser-1179 phosphorylation. Shown are the results of eNOS activity assays and eNOS Ser-1179 phosphorylation determinations performed in BAEC incubated with VEGF (10 ng/ml for 90 min) or vehicle, as indicated, then treated with S1P (100 nM for 5 min). Open bars show the results of eNOS activity assays: NOS activity was quantitated as the formation of l-[3H]citrulline from l-[3H]arginine as described in detail in Experimental Procedures. Each data point represents the mean ± SEM derived from four independent cell preparations, each analyzed in triplicate. Hatched bars show the results of eNOS Ser-1179 phosphorylation measurements assessed by densitometric analyses of immunoblots probed with a phosphorylation state-specific antibody (see representative experiment in Fig. 4). The data shown are pooled from four independent experiments, in which the fold increase in eNOS Ser-1179 phosphorylation at 5 min after the addition of S1P is determined relative to the signals obtained in the absence of VEGF and S1P; data are represented as the mean ± SEM. *, P < 0.05, compared with values obtained in the absence of S1P or VEGF; †, P < 0.05, compared with values determined in cells not incubated with VEGF.
Fig. 6.
Effects of calphostin C on VEGF-induced enhancement of S1P-mediated eNOS Ser-1179 phosphorylation. Shown are the results of immunoblots probed with antibodies directed against S1P1 receptors, eNOS Ser-1179 phosphorylated eNOS (p-eNOS), or total eNOS, as indicated. BAEC were pretreated with calphostin C (400 nM for 30 min) or vehicle, followed by VEGF (10 ng/ml for 90 min) or vehicle, then treated with S1P (100 nM for 5 min). Cell lysates (20μg per lane) were resolved by SDS/PAGE, transferred to a nitrocellulose membrane, and probed with antibodies as indicated. Equal loading of samples was confirmed by reprobing the immunoblots with antibodies against (total) eNOS. Shown are representative data from an experiment that was independently repeated three times with equivalent results.
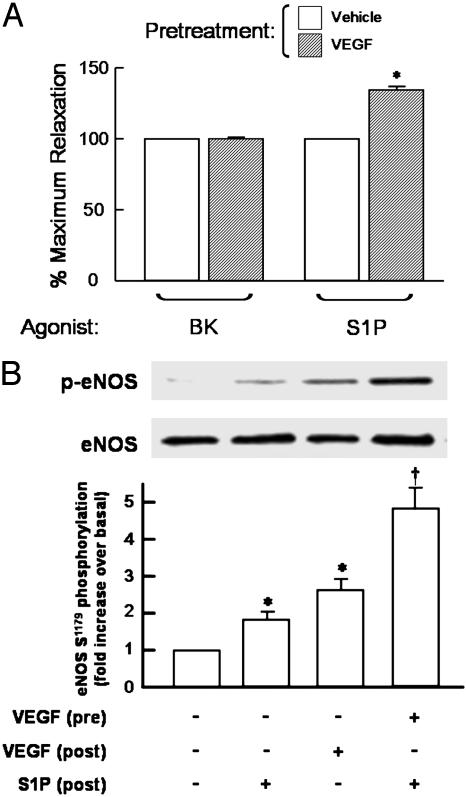
We have recently shown that S1P induces eNOS-dependent vasorelaxation in isolated rat mesenteric arterioles (14), and we therefore extended our studies in cultured endothelial cells to explore the effects of pretreatment with VEGF on the responses of isolated blood vessels to S1P. As shown in Fig. 7A, pretreatment of isolated arterioles with VEGF specifically and markedly augments the subsequent vasorelaxation response to S1P, but VEGF pretreatment does not alter the vessel's response to the classic eNOS agonist bradykinin. The effects of VEGF on augmenting S1P-mediated vasodilation were not seen when the agonists were added simultaneously; rather, preincubation of the vessel with VEGF for 60–90 min was necessary to observe the enhanced S1P vasodilation response (data not shown). The experiment shown in Fig. 7B demonstrates that pretreatment with VEGF also significantly potentiates S1P-induced eNOS phosphorylation at Ser-1179, relative to the S1P response observed in arteries preincubated with vehicle. These data demonstrate that VEGF pretreatment potentiates S1P-mediated responses in ex vivo blood vessel preparations as well as in cultured endothelial cells.
Fig. 7.
Effects of VEGF pretreatment on S1P-induced vascular responses. (A) The effects of VEGF pretreatment on subsequent agonist-induced vasodilation responses. Rat mesenteric arteries, preconstricted with norepinephrine, were at first treated with bradykinin (BK, 1μM) or S1P (100 nM). The degrees of maximum relaxation were recorded, and the vessels were allowed to recover to baseline. Note that S1P and bradykinin induce similar degrees of eNOS-dependent vasorelaxation in these arteriole preparations in the absence of VEGF pretreatment (14). The vessels were then incubated with VEGF (20 ng/ml for 90 min; hatched bars) or vehicle (open bars), and then treated for 5 min with bradykinin or S1P. To quantitate the effects of VEGF pretreatment on subsequent vasodilation responses, the level of agonist-induced vasorelaxation determined after incubation with VEGF (or vehicle) was determined and then normalized to the vasodilation response seen with the same agonist before incubating the vessel with VEGF (or vehicle). Data are expressed as the percentage of maximum relaxation induced by each agonist, normalized to the maximal response evoked by the respective agonist stimulation before incubation with VEGF or vehicle. Each data point represents mean ± SEM derived from pooled data of four to six experiments. *, P < 0.05, compared with values obtained in the absence of pretreatments. (B) The effects of VEGF treatment on S1P-mediated Ser-1179 eNOS phosphorylation in intact vessels. (Upper) The results of immunoblots analyzed in segments of thoracic aorta (1 cm) probed with antibodies against eNOS phosphoserine-1179 or total eNOS, as indicated. The arteries were incubated with VEGF (20 ng/ml for 90 min) or vehicle, then treated with S1P (100 nM) or VEGF (20 ng/ml) for 5 min, immediately processed for immunoblot analyses, and probed with antibodies as indicated. A representative immunoblot is shown. (Lower) A bar graph presenting the results of densitometric analyses from pooled data, plotted as the fold increase in eNOS Ser-1179 phosphorylation relative to the signal obtained in the absence of drug treatment. Each data point represents the mean ± SEM derived from four independent experiments. *, P < 0.01, compared with values determined in the absence of agonist; †, P < 0.01, comparing S1P-mediated eNOS phosphorylation in vehicle vs. VEGF-treated arteries.
Discussion
These studies establish that VEGF promotes the rapid, reversible, and concentration-dependent expression of S1P1 receptor protein and mRNA in endothelial cells (Figs. 1 and 2). The EC50 of 2–5 ng/ml observed for the VEGF-induced increase in S1P1 protein (Fig. 1B) and mRNA (Fig. 2B) is within the physiologically relevant concentration range of this growth factor (16). In contrast to the robust response seen with VEGF, we found that neither S1P nor a variety of other growth factors, including basic fibroblast growth factor and hepatocyte growth factor, had any effect on S1P1 expression under these conditions (data not shown). Although diverse growth factors may influence transcriptional pathways in endothelial cells (30), our results suggest that VEGF may be unique in its ability to induce S1P1 expression in this experimental system. VEGF-induced S1P1 expression is seen within 30 min of VEGF addition, a time course consistent with that previously observed for PMA-induced S1P1 gene regulation (20). This is a strikingly rapid time course for induction of G protein-coupled receptors in response to an extracellular signal and suggests that S1P1 receptor expression may be subject to dynamic regulation by VEGF under (patho)physiological conditions. Significantly, VEGF did not alter the amount of other signaling proteins in the time frame of these studies: neither eNOS, Akt, bradykinin B2 receptors, nor caveolin-1 were up-regulated by VEGF treatment (Fig. 1). Although VEGF may modulate expression of other signaling proteins over a much longer time frame (30–32), the fact that S1P1 levels are acutely altered by VEGF (Figs. 1 and 2) suggests that S1P1 induction by VEGF and the consequent potentiation of S1P-mediated responses may provide a mechanism for shorter-term regulation of endothelial responses.
The principal VEGF receptor in endothelial cells, termed KDR (16, 32), is a receptor tyrosine kinase that also modulates several distal serine/threonine protein kinases. We used a series of protein kinase inhibitors to explore the mechanisms whereby VEGF induces expression of S1P1. The tyrosine kinase inhibitor genistein, which inhibits a broad range of tyrosine kinases, completely blocked the VEGF-induced increase in S1P1 mRNA (Fig. 3A). Genistein also blocks VEGF-induced phosphorylation of the serine/threonine kinase Akt (Fig. 3B), indicating that protein tyrosine kinase-dependent pathways may lie upstream of VEGF-mediated Akt activation. By contrast, genistein has no effect on S1P-mediated Akt activation (Fig. 3B), suggesting that key S1P1-mediated responses are largely independent of protein tyrosine kinase pathways, as previously reported (7). These observations are at odds with a recent report (33) that suggested that S1P1 responses are entirely the consequence of S1P-mediated tyrosine phosphorylation of the VEGF receptor, KDR, in endothelial cells. This intriguing hypothesis contrasts with our current (Fig. 3) and previous studies, which document that S1P and VEGF elicit distinct protein kinase signaling pathways in endothelial cells. The recent proposal (33) that S1P elicits its cellular responses through KDR activation is largely based on observations that down-regulation of KDR expression (using antisense approaches) leads to an attenuation of S1P-mediated responses in endothelial cells. In light of our current findings, we suggest that these observations might be completely reinterpreted. Because VEGF markedly potentiates S1P-mediated responses (Figs. 4, 5, 6, 7) through induction of S1P1 expression (Figs. 1,2,3), we would anticipate that down-regulation of the VEGF receptor KDR would prevent VEGF-promoted S1P1 induction and thereby attenuate S1P-mediated responses. Rather than S1P1 responses being solely mediated by KDR transactivation, as proposed (33), we suggest that VEGF-mediated S1P1 induction serves to explain the principal form of “cross-talk” between these two important signaling pathways.
The intracellular signaling pathways that lead to S1P1 gene induction were explored by using pharmacological approaches with a series of protein kinase inhibitors. VEGF-mediated S1P1 induction was blocked by the tyrosine kinase inhibitor genistein (discussed above) as well as by the PKC inhibitor calphostin C (Fig. 4).
In contrast, neither the PI3-K inhibitor wortmannin nor the MAP kinase pathway inhibitor PD98059 blocked VEGF-mediated induction of S1P1 mRNA. These findings suggest that signaling pathways involving protein tyrosine kinases and/or PKC, but not PI3-K/Akt or MAP kinase pathways, are involved in the regulatory mechanisms whereby VEGF induces S1P1 expression. Protein kinase C pathways had been previously implicated in S1P1 induction, dating back to the original discovery of the S1P1 receptor as an immediate early gene induced on treatment of endothelial cells with the phorbol ester PMA (20). VEGF has been previously observed to activate PKC pathways, especially via its β-isoform, in endothelial cells (29); hemodynamic shear stress also activates PKC and leads to S1P1 induction (34). Taken together, these findings suggest that PKC pathways may play a key role in the regulation of S1P1 gene expression.
The VEGF-induced increase in S1P1 receptor expression leads to enhanced cellular responses to subsequent S1P treatment. The VEGF-induced S1P1 receptors thus seem to be functionally coupled, as revealed by marked increases in S1P-mediated eNOS activation in cells treated with VEGF (Fig. 5), as well as increased S1P-promoted phosphorylation of eNOS, Akt, and ERK1/2 (Fig. 4). When VEGF-mediated S1P1 induction is blocked by calphostin C, these enhanced S1P-modulated responses are also lost (Fig. 6). Furthermore, pretreatment with VEGF induces significantly higher degrees of vasorelaxation (Fig. 7A) as well as phosphorylation of eNOS at Ser-1179 (Fig. 7B) in isolated rat arterial vessels. Although it is possible that VEGF may lead to the induction of other members of the family of lipid-activated G protein-coupled receptors, the predominant S1P receptor subtype expressed in these cells seems to be S1P1 (11, 17, 19, 35). Furthermore, VEGF failed to alter the expression of either S1P2 (EDG-5) or S1P3 (EDG-3) receptor protein (data not shown). Certainly, the S1P1 receptor subtype has been clearly implicated in eNOS activation (5–7), leading to a broad range of cellular responses, including inhibition of apoptosis (12, 13), vasorelaxation (14), and angiogenesis (15). Furthermore, S1P and polypeptide growth factors including VEGF exert synergistic actions to modulate angiogenesis (17). Thus, based on our current observations, it is intriguing to speculate that VEGF-mediated induction of S1P1 proteins, which leads to the augmentation of eNOS responses to S1P, may represent a mechanism for the synergistic activation of signaling pathways elicited by G protein-coupled S1P/EDG and tyrosine kinase-coupled VEGF pathways in vascular endothelial cells. It is also noteworthy that many of these molecules that modulate angiogenic responses of endothelial cells are specifically enriched in plasmalemmal caveolae: these include scaffolding proteins of caveolae, caveolin-1 (36), receptors such as S1P1 (5), intervening molecules such as G proteins (37), PI3-K (38), and c-Src tyrosine kinases (39), as well as effectors such as eNOS (40). Some of these molecules are also subject to gene regulation by VEGF (refs. 30, 31, and current studies). Although subcellular targeting of newly synthesized molecules after VEGF stimulation remains less well understood, these studies, when taken together, suggest that gene regulation of those molecules specifically enriched in plasmalemmal caveolae may also represent an important point of control in the regulation of angiogenesis and other vascular responses in endothelial cells.
There have been numerous recent reports describing the transactivation of receptor tyrosine kinases by G protein-coupled receptors, and of G protein-coupled receptors by tyrosine kinases. For example, the stimulation of β2-adrenergic receptors in cardiac myocytes leads to transactivation of EGF receptors (41), ultimately leading to hypertrophic responses of these cells (42). For S1P/EDG pathways, it was originally postulated that S1P, which is produced after stimulation with platelet-derived growth factor (PDGF), acts as an intracellular second messenger in NIH 3T3 cells (23). However, a subsequent observation by the same group revealed that S1P is released into the extracellular space after PDGF stimulation, thereby leading to transactivation of S1P1 receptors and ultimately to the augmentation of motility in transfected 3T3 cells (43). It remains to be established whether or not endothelial cells also release S1P after stimulation with growth factor stimulation. If this is the case with VEGF, S1P release into extracellular space may synergistically modulate S1P1 receptors, which are markedly induced after VEGF stimulation (Figs. 2 and 3). The current studies describe the strikingly rapid induction of G protein-coupled receptors by stimulation with polypeptide growth factors within vascular endothelial cells.
These experiments have shown that the angiogenic polypeptide growth factor VEGF induces the expression of S1P1 receptors, which in turn are activated by the platelet-derived sphingolipid S1P in endothelial cells. Because endothelial cells, as well as isolated arteries incubated with VEGF, exhibit an enhanced biological response to S1P, the induction of S1P1 by VEGF may represent important point of vascular regulation by this growth factor. We propose that induction of G protein-coupled S1P1 receptors by tyrosine kinase-coupled VEGF receptors may identify a novel mode of “transactivation” of receptor-dependent signal transduction pathways that regulate eNOS activity and other important biological responses in the vascular wall.
Acknowledgments
We thank Drs. Timothy Hla and Roger A. Sabbadini for providing cDNA plasmids encoding EDG-1 (S1P1) and anti-EDG-1 (S1P1) antibody, respectively. We are grateful to Dr. Thomas Anger (Brigham and Women's Hospital) for many helpful discussions in performing Northern blot analysis. This work was supported by a grant to T.M. from the National Institutes of Health (RO-1 HL46457) and by a Howard Hughes Medical Institute predoctoral fellowship (to P.A.E.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: eNOS, endothelial isoform of NO synthase; VEGF, vascular endothelial growth factor; S1P, sphingosine 1-phosphate; PI3-K, phosphoinositide 3-kinase; PMA, phorbol 12-myristate 13-acetate; BAEC, bovine aortic endothelial cells; MAP, mitogen-activated protein.
References
- 1.Loscalzo, J. & Welch, G. (1995) Prog. Cardiovasc. Dis. 38, 87–104. [DOI] [PubMed] [Google Scholar]
- 2.Mombouli, J. V. & Vanhoutte, P. M. (1995) Annu. Rev. Pharmacol. Toxicol. 35, 679–705. [DOI] [PubMed] [Google Scholar]
- 3.Ku, D. D., Zaleski, J. K., Liu, S. & Brock, T. A. (1993) Am. J. Physiol. 265, H586–H592. [DOI] [PubMed] [Google Scholar]
- 4.Kuchan, M. J. & Frangos, J. A. (1994) Am. J. Physiol. 266, C628–C636. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi, J. & Michel, T. (2000) J. Biol. Chem. 275, 32363–32370. [DOI] [PubMed] [Google Scholar]
- 6.Igarashi, J., Bernier, S. G. & Michel, T. (2001) J. Biol. Chem. 276, 12420–12426. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi, J. & Michel, T. (2001) J. Biol. Chem. 276, 36281–36288. [DOI] [PubMed] [Google Scholar]
- 8.Hla, T., Lee, M. J., Ancellin, N., Liu, C. H., Thangada, S., Thompson, B. D. & Kluk, M. (1999) Biochem. Pharmacol. 58, 201–207. [DOI] [PubMed] [Google Scholar]
- 9.Hla, T., Lee, M. J., Ancellin, N., Paik, J. H. & Kluk, M. J. (2001) Science 294, 1875–1878. [DOI] [PubMed] [Google Scholar]
- 10.Muraki, K. & Imaizumi, Y. (2001) J. Physiol. 537, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, H., Goetzl, E. J. & An, S. (2000) Am. J. Physiol. 278, C612–C618. [DOI] [PubMed] [Google Scholar]
- 12.Kwon, Y. G., Min, J. K., Kim, K. M., Lee, D. J., Billiar, T. R. & Kim, Y. M. (2001) J. Biol. Chem. 276, 10627–10633. [DOI] [PubMed] [Google Scholar]
- 13.Kimura, T., Sato, K., Kuwabara, A., Tomura, H., Ishiwara, M., Kobayashi, I., Ui, M. & Okajima, F. (2001) J. Biol. Chem. 276, 31780–31785. [DOI] [PubMed] [Google Scholar]
- 14.Dantas, A. P., Igarashi, J. & Michel, T. (2003) Am. J. Physiol. 284, H2045–H2052. [DOI] [PubMed] [Google Scholar]
- 15.Rikitake, Y., Hirata, K., Kawashima, S., Ozaki, M., Takahashi, T., Ogawa, W., Inoue, N. & Yokoyama, M. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 108–114. [DOI] [PubMed] [Google Scholar]
- 16.Petrova, T. V., Makinen, T. & Alitalo, K. (1999) Exp. Cell Res. 253, 117–130. [DOI] [PubMed] [Google Scholar]
- 17.Lee, M. J., Thangada, S., Claffey, K. P., Ancellin, N., Liu, C. H., Kluk, M., Volpi, M., Sha'afi, R. I. & Hla, T. (1999) Cell 99, 301–312. [DOI] [PubMed] [Google Scholar]
- 18.Murohara, T., Asahara, T., Silver, M., Bauters, C., Masuda, H., Kalka, C., Kearney, M., Chen, D., Symes, J. F., Fishman, M. C., et al. (1998) J. Clin. Invest. 101, 2567–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, F., Van Brocklyn, J. R., Hobson, J. P., Movafagh, S., Zukowska-Grojec, Z., Milstien, S. & Spiegel, S. (1999) J. Biol. Chem. 274, 35343–35350. [DOI] [PubMed] [Google Scholar]
- 20.Hla, T. & Maciag, T. (1990) J. Biol. Chem. 265, 9308–9313. [PubMed] [Google Scholar]
- 21.Nakajima, N., Cavalli, A. L., Biral, D., Glembotski, C. C., McDonough, P. M., Ho, P. D., Betto, R., Sandona, D., Palade, P. T., Dettbarn, C. A., et al. (2000) Eur. J. Biochem. 267, 5679–5686. [DOI] [PubMed] [Google Scholar]
- 22.Lee, M. J., Evans, M. & Hla, T. (1996) J. Biol. Chem. 271, 11272–11279. [DOI] [PubMed] [Google Scholar]
- 23.Olivera, A. & Spiegel, S. (1993) Nature 365, 557–560. [DOI] [PubMed] [Google Scholar]
- 24.Bernier, S. G., Haldar, S. & Michel, T. (2000) J. Biol. Chem. 275, 30707–30715. [DOI] [PubMed] [Google Scholar]
- 25.Busconi, L. & Michel, T. (1993) J. Biol. Chem. 268, 8410–8413. [PubMed] [Google Scholar]
- 26.Ishii, I., Friedman, B., Ye, X., Kawamura, S., McGiffert, C., Contos, J. J., Kingsbury, M. A., Zhang, G., Brown, J. H. & Chun, J. (2001) J. Biol. Chem. 276, 33697–33704. [DOI] [PubMed] [Google Scholar]
- 27.Blum, S., Issbruker, K., Willuweit, A., Hehlgans, S., Lucerna, M., Mechtcheriakova, D., Walsh, K., von der Ahe, D., Hofer, E. & Clauss, M. (2001) J. Biol. Chem. 276, 33428–33434. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi, T., Ueno, H. & Shibuya, M. (1999) Oncogene 18, 2221–2230. [DOI] [PubMed] [Google Scholar]
- 29.Xia, P., Aiello, L. P., Ishii, H., Jiang, Z. Y., Park, D. J., Robinson, G. S., Takagi, H., Newsome, W. P., Jirousek, M. R. & King, G. L. (1996) J. Clin. Invest. 98, 2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, J., Razani, B., Tang, S., Terman, B. I., Ware, J. A. & Lisanti, M. P. (1999) J. Biol. Chem. 274, 15781–15785. [DOI] [PubMed] [Google Scholar]
- 31.Shen, B. Q., Lee, D. Y. & Zioncheck, T. F. (1999) J. Biol. Chem. 274, 33057–33063. [DOI] [PubMed] [Google Scholar]
- 32.Shen, B. Q., Lee, D. Y., Gerber, H. P., Keyt, B. A., Ferrara, N. & Zioncheck, T. F. (1998) J. Biol. Chem. 273, 29979–29985. [DOI] [PubMed] [Google Scholar]
- 33.Tanimoto, T., Jin, Z. G. & Berk, B. C. (2002) J. Biol. Chem. 277, 42997–43001. [DOI] [PubMed] [Google Scholar]
- 34.Takada, Y., Kato, C., Kondo, S., Korenaga, R. & Ando, J. (1997) Biochem. Biophys. Res. Commun. 240, 737–741. [DOI] [PubMed] [Google Scholar]
- 35.Morales-Ruiz, M., Lee, M. J., Zoellner, S., Gratton, J. P., Scotland, R., Shiojima, I., Walsh, K., Hla, T. & Sessa, W. C. (2001) J. Biol. Chem. 276, 19672–19677. [DOI] [PubMed] [Google Scholar]
- 36.Shaul, P. W. & Anderson, R. G. W. (1998) Am. J. Physiol. 275, L843–L851. [DOI] [PubMed] [Google Scholar]
- 37.Oh, P. & Schnitzer, J. E. (2001) Mol. Biol. Cell 12, 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zundel, W., Swiersz, L. M. & Giaccia, A. (2000) Mol. Cell. Biol. 20, 1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, S., Seitz, R. & Lisanti, M. P. (1996) J. Biol. Chem. 271, 3863–3868. [PubMed] [Google Scholar]
- 40.Shaul, P. W., Smart, E. J., Robinson, L. J., German, Z., Yuhanna, I. S., Ying, Y., Anderson, R. G. W. & Michel, T. (1996) J. Biol. Chem. 271, 6518–6522. [DOI] [PubMed] [Google Scholar]
- 41.Maudsley, S., Pierce, K. L., Zamah, A. M., Miller, W. E., Ahn, S., Daaka, Y., Lefkowitz, R. J. & Luttrell, L. M. (2000) J. Biol. Chem. 275, 9572–9580. [DOI] [PubMed] [Google Scholar]
- 42.Asakura, M., Kitakaze, M., Takashima, S., Liao, Y., Ishikura, F., Yoshinaka, T., Ohmoto, H., Node, K., Yoshino, K., Ishiguro, H., et al. (2002) Nat. Med. 8, 35–40. [DOI] [PubMed] [Google Scholar]
- 43.Hobson, J. P., Rosenfeldt, H. M., Barak, L. S., Olivera, A., Poulton, S., Caron, M. G., Milstien, S. & Spiegel, S. (2001) Science 291, 1800–1803. [DOI] [PubMed] [Google Scholar]