Abstract
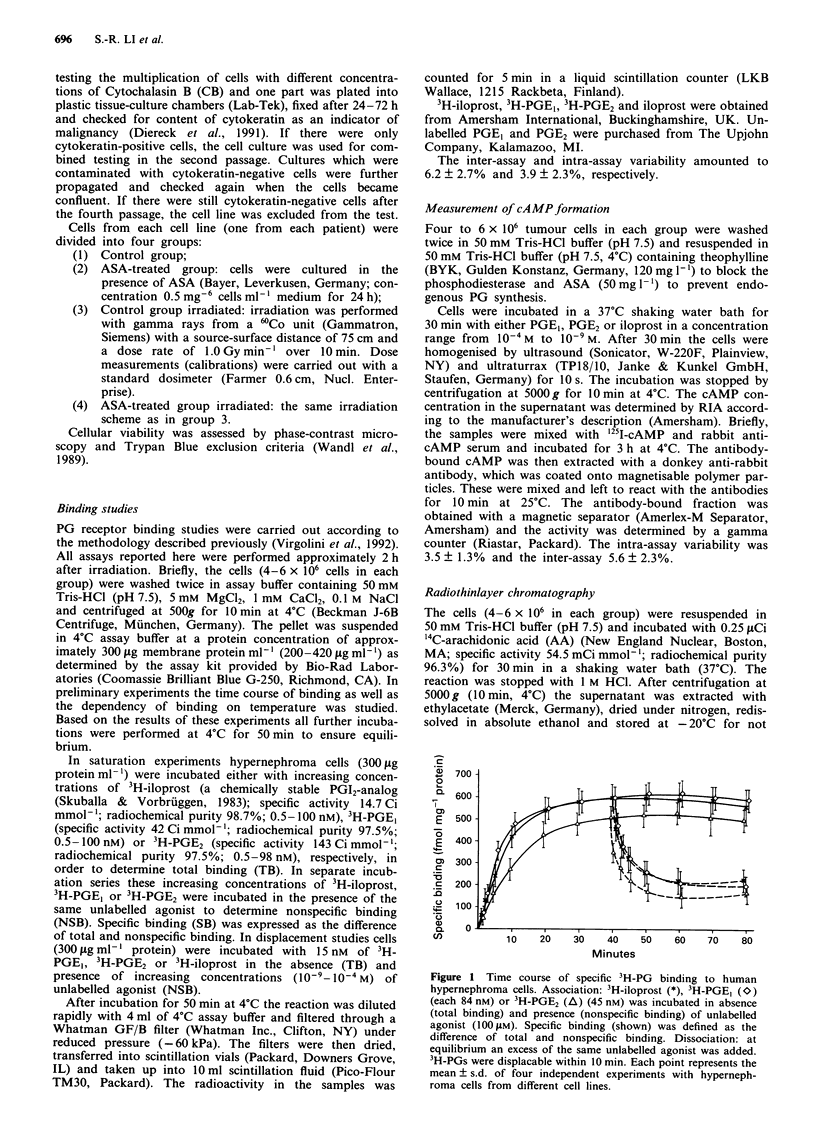
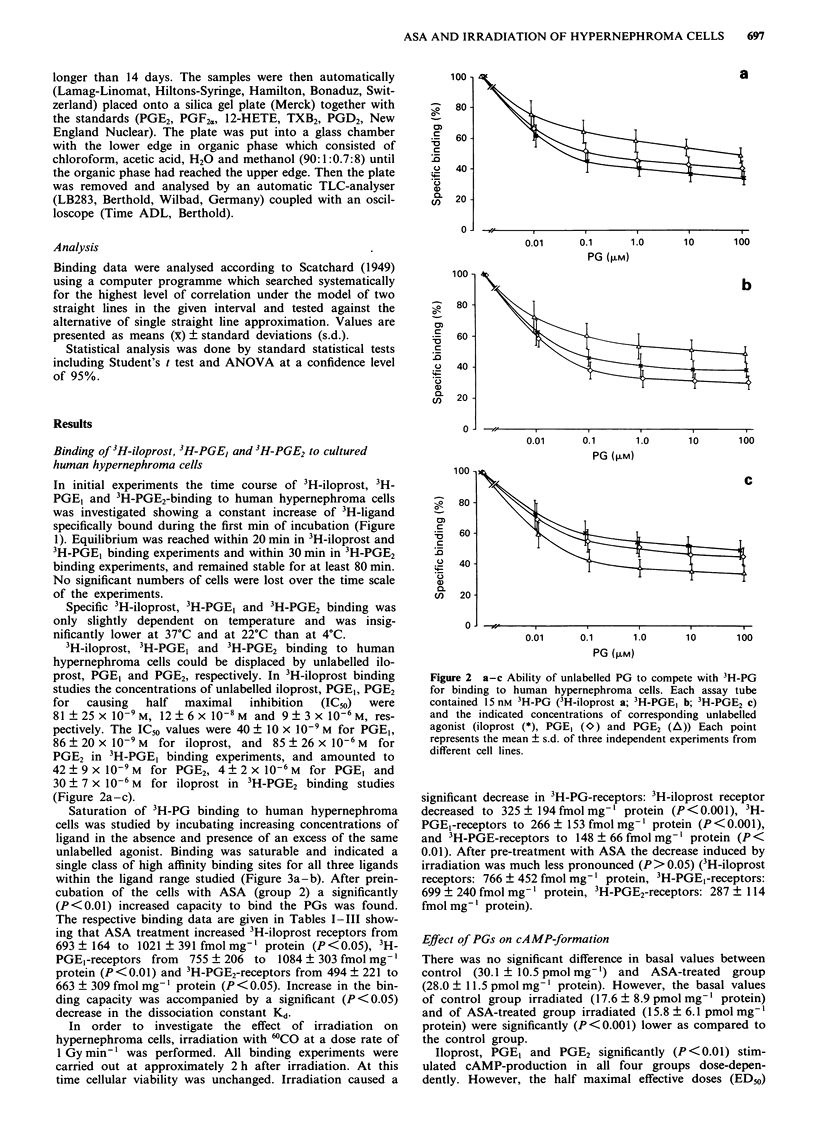
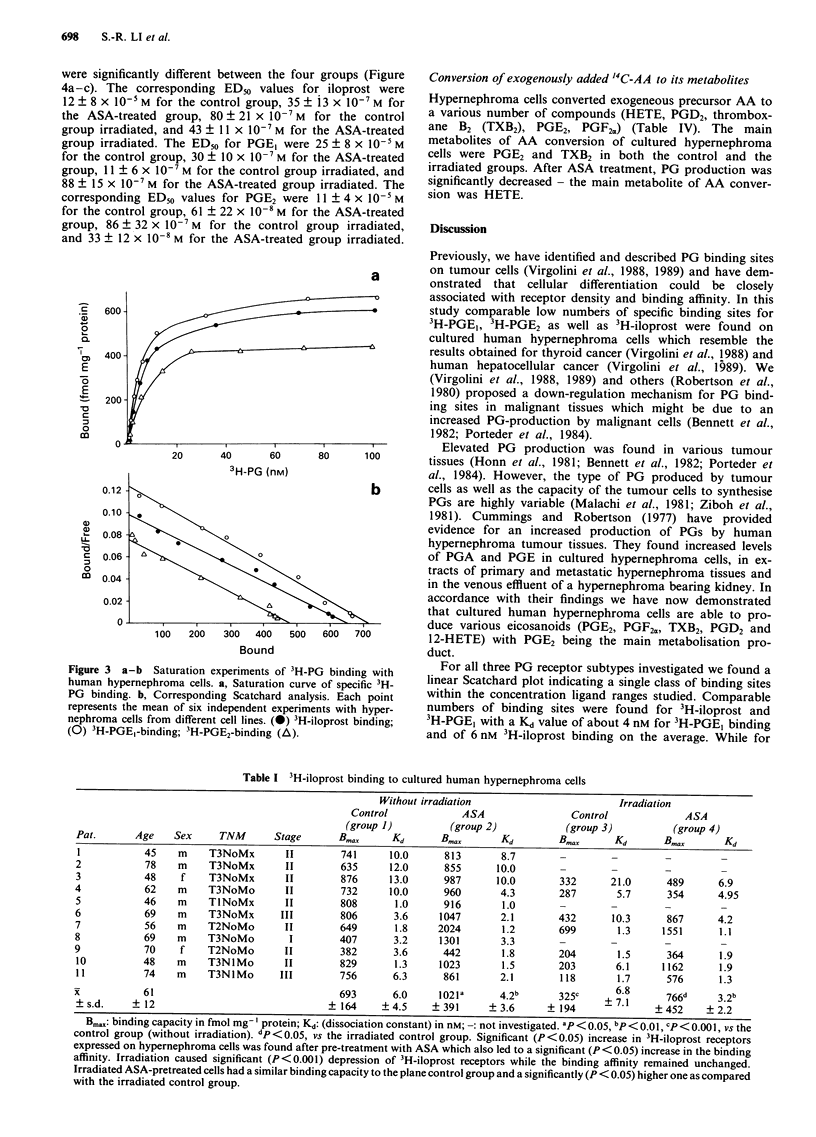
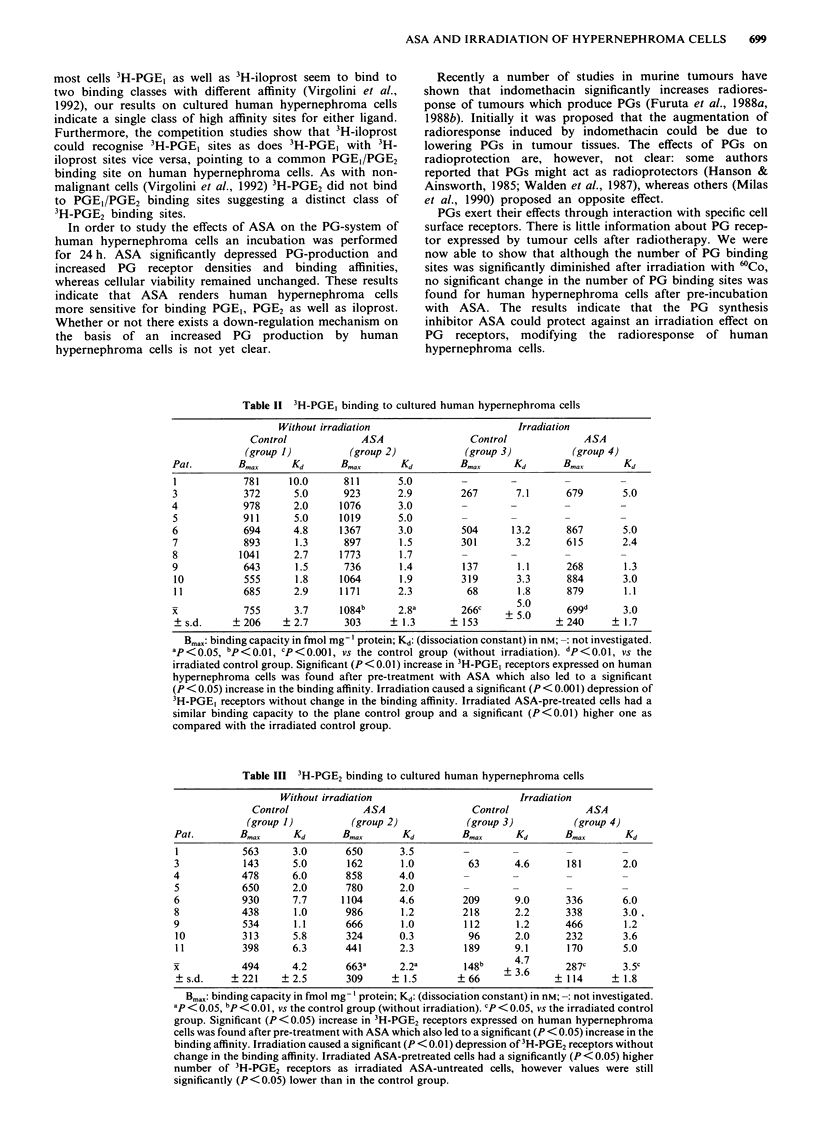
There is abundant evidence that inhibitors of prostaglandin (PG) biosynthesis might increase the radioresponse of certain tumour cells. This study investigated specific PG binding sites, eicosanoid production as well as intracellular cAMP levels in cultured human hypernephroma cells derived from 11 patients upon nephrectomy. Scatchard analyses of the binding data revealed specific PGE1-, PGE2- as well as PGI2-binding sites (PGE1: Bmax = 755 +/- 206 fmol mg-1 protein, Kd = 3.7 +/- 2.7 nM PGE2: Bmax = 494 +/- 221 fmol mg-1 protein, Kd = 4.2 +/- 2.5 nM; PGI2: Bmax = 693 +/- 164 fmol mg-1 protein, Kd = 6.0 +/- 4.5 nM). Significant (P < 0.01) increase in PG binding sites expressed on human hypernephroma cells (PGE1: Bmax = 1084 +/- 303 fmol mg-1 protein, Kd = 2.8 +/- 1.3 nM; PGE2: Bmax = 663 +/- 309 fmol mg-1 protein, Kd = 2.2 +/- 1.5 nM; PGI2: Bmax = 1021 +/- 391 fmol/protein, Kd = 4.2 +/- 3.6 nM) and inhibition of PG biosynthesis (TXB2: -82.5%, PGE2: -87.5%. PGD2: -80.6%, PGF2: -81.3%) were found after acetylsalicylic acid (ASA)-treatment (0.5 mg 10(-6) cells for 24 h). Following irradiation (60Co, 1.0 Gy/min-1 over 10(min), PG binding sites (PGE1: Bmax = 266 +/- 153 fmol mg-1 protein, Kd = 5.0 +/- 5.0 nM; PGE2: Bmax = 148 +/- 66 fmol mg-1 protein, Kd = 4.7 +/- 3.6 nM; PGI2: Bmax = 325 +/- 194 fmol mg-1 protein, Kd = 6.8 +/- 7.1 nM) were significantly (P < 0.01) diminished. However, irradiation had no significant effect on PG binding sites in ASA-pretreated cells (PGE1: Bmax = 699 +/- 240 fmol mg-1 protein, Kd = 3.5 +/- 1.8 nM; iloprost: Bmax = 766 +/- 452 fmol mg-1 protein, Kd = 3.2 +/- 2.2 nM). Although there was no significant difference in the basal values for cAMP between control and ASA-treated group cells, the PG-induced cAMP-production was less pronounced in the control group. Taken together, the findings suggest that ASA may modify the radioresponse of cultured human hypernephroma cells by preventing the decrease of PG binding sites induced by irradiation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A., Carroll M. A., Stamford I. F., Whimster W. F., Williams F. Prostaglandins and human lung carcinomas. Br J Cancer. 1982 Dec;46(6):888–893. doi: 10.1038/bjc.1982.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun M., Hoffmann M. K. Combination immunotherapy of cancer in a mouse model: synergism between tumor necrosis factor and other defense systems. Cancer Res. 1987 Jan 1;47(1):115–118. [PubMed] [Google Scholar]
- Cummings K. B., Robertson R. P. Prostaglandin: increased production by renal cell carcinoma. J Urol. 1977 Nov;118(5):720–723. doi: 10.1016/s0022-5347(17)58172-0. [DOI] [PubMed] [Google Scholar]
- Dierick A. M., Praet M., Roels H., Verbeeck P., Robyns C., Oosterlinck W. Vimentin expression of renal cell carcinoma in relation to DNA content and histological grading: a combined light microscopic, immunocytochemical and cytophotometrical analysis. Histopathology. 1991 Apr;18(4):315–322. doi: 10.1111/j.1365-2559.1991.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Furuta Y., Hall E. R., Sanduja S., Barkley T., Jr, Milas L. Prostaglandin production by murine tumors as a predictor for therapeutic response to indomethacin. Cancer Res. 1988 Jun 1;48(11):3002–3007. [PubMed] [Google Scholar]
- Furuta Y., Hunter N., Barkley T., Jr, Hall E., Milas L. Increase in radioresponse of murine tumors by treatment with indomethacin. Cancer Res. 1988 Jun 1;48(11):3008–3013. [PubMed] [Google Scholar]
- Goldberg M. L., Burke G. C., Morris H. P. Cyclic AMP and cyclic GMP content and binding in malignancy. Biochem Biophys Res Commun. 1975 Jan 20;62(2):320–327. doi: 10.1016/s0006-291x(75)80141-0. [DOI] [PubMed] [Google Scholar]
- Halperin E. C., Harisiadis L. The role of radiation therapy in the management of metastatic renal cell carcinoma. Cancer. 1983 Feb 15;51(4):614–617. doi: 10.1002/1097-0142(19830215)51:4<614::aid-cncr2820510411>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hanson W. R., Ainsworth E. J. 16,16-Dimethyl prostaglandin E2 induces radioprotection in murine intestinal and hematopoietic stem cells. Radiat Res. 1985 Aug;103(2):196–203. [PubMed] [Google Scholar]
- Heidrick M. L., Ryan W. L. Adenosine 3',5'-cyclic monophosphate and contact inhibition. Cancer Res. 1971 Sep;31(9):1313–1315. [PubMed] [Google Scholar]
- Honn K. V., Bockman R. S., Marnett L. J. Prostaglandins and cancer: a review of tumor initiation through tumor metastasis. Prostaglandins. 1981 May;21(5):833–864. doi: 10.1016/0090-6980(81)90240-9. [DOI] [PubMed] [Google Scholar]
- Küng W., Bechtel E., Geyer E., Salokangas A., Preisz J., Huber P., Torhorst J., Jungmann R. A., Talmadge K., Eppenberger U. Altered levels of cyclic nucleotides, cyclic AMP phosphodiesterase and adenylyl cyclase activities in normal, dysplastic and neoplastic human mammary tissue. FEBS Lett. 1977 Oct 1;82(1):102–106. doi: 10.1016/0014-5793(77)80895-8. [DOI] [PubMed] [Google Scholar]
- Lang E. K., deKernion J. B. Transcatheter embolization of advanced renal cell carcinoma with radioactive seeds. J Urol. 1981 Nov;126(5):581–586. doi: 10.1016/s0022-5347(17)54635-2. [DOI] [PubMed] [Google Scholar]
- Lynch N. R., Castes M., Astoin M., Salomon J. C. Mechanism of inhibition of tumour growth by aspirin and indomethacin. Br J Cancer. 1978 Oct;38(4):503–512. doi: 10.1038/bjc.1978.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malachi T., Chaimoff C., Feller N., Halbrecht I. Prostaglandin E2 and cyclic AMP in tumor and plasma of breast cancer patients. J Cancer Res Clin Oncol. 1981;102(1):71–79. doi: 10.1007/BF00410536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milas L., Furuta Y., Hunter N., Nishiguchi I., Runkel S. Dependence of indomethacin-induced potentiation of murine tumor radioresponse on tumor host immunocompetence. Cancer Res. 1990 Aug 1;50(15):4473–4477. [PubMed] [Google Scholar]
- Minton J. P., Matthews R. H., Wisenbaugh T. W. Elevated adenosine 3',5'-cyclic monophosphate levels in human and animal tumors in vivo. J Natl Cancer Inst. 1976 Jul;57(1):39–41. doi: 10.1093/jnci/57.1.39. [DOI] [PubMed] [Google Scholar]
- Porteder H., Matejka M., Ulrich W., Sinzinger H. The cyclo-oxygenase and lipoxygenase pathways in human oral cancer tissue. J Maxillofac Surg. 1984 Aug;12(4):145–147. doi: 10.1016/s0301-0503(84)80234-9. [DOI] [PubMed] [Google Scholar]
- Powles T. J., Alexander P., Millar J. L. Enhancement of anti-cancer activity of cytotoxic chemotherapy with protection of normal tissues by inhibition of P.G. synthesis. Biochem Pharmacol. 1978 May 1;27(9):1389–1392. doi: 10.1016/0006-2952(78)90127-2. [DOI] [PubMed] [Google Scholar]
- Robertson R. P., Baylink D. J., Marini B. J., Adkison H. W. Elevated prostaglandins and suppressed parathyroid hormone associated with hypercalcemia and renal cell carcinoma. J Clin Endocrinol Metab. 1975 Jul;41(1):164–167. doi: 10.1210/jcem-41-1-164. [DOI] [PubMed] [Google Scholar]
- Robertson R. P., Westcott K. R., Storm D. R., Rice M. G. Down-regulation in vivo of PGE receptors and adenylate cyclase stimulation. Am J Physiol. 1980 Jul;239(1):E75–E80. doi: 10.1152/ajpendo.1980.239.1.E75. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Difference in the cyclic adenosine 3',5'-monophosphate levels in normal and transformed cells. Nat New Biol. 1972 Mar 1;236(61):14–16. doi: 10.1038/newbio236014a0. [DOI] [PubMed] [Google Scholar]
- Skuballa W., Vorbrüggen H. Synthesis of ciloprost (ZK 36 374): a chemically stable and biologically potent prostacyclin analog. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:299–305. [PubMed] [Google Scholar]
- Stevens R. H., Loven D. P., Osborne J. W., Prall J. L. Cyclic nucleotide concentrations in 1,2-dimethylhydrazine and x-ray induced rat small intestinal cancer. Cancer Lett. 1978 Jun;4(6):325–332. doi: 10.1016/s0304-3835(78)95557-x. [DOI] [PubMed] [Google Scholar]
- Virgolini I., Hermann M., Sinzinger H. Decrease of prostaglandin I2 binding sites in thyroid cancer. Br J Cancer. 1988 Nov;58(5):584–588. doi: 10.1038/bjc.1988.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini I., Li S., Sillaber C., Majdic O., Sinzinger H., Lechner K., Bettelheim P., Valent P. Characterization of prostaglandin (PG)-binding sites expressed on human basophils. Evidence for a prostaglandin E1, I2, and a D2 receptor. J Biol Chem. 1992 Jun 25;267(18):12700–12708. [PubMed] [Google Scholar]
- Virgolini I., Sinzinger H., Müller C., Hermann M. Human hepatocellular cancers show decreased prostaglandin E1 binding capacity. Br J Cancer. 1989 Mar;59(3):407–409. doi: 10.1038/bjc.1989.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden T. L., Jr, Patchen M., Snyder S. L. 16,16-Dimethyl prostaglandin E2 increases survival in mice following irradiation. Radiat Res. 1987 Mar;109(3):440–448. [PubMed] [Google Scholar]
- Wandl E. O., Ono K., Kain R., Herbsthofer T., Hienert G., Höbarth K. Linear correlation between surviving fraction and the micronucleus frequency. Int J Radiat Biol. 1989 Nov;56(5):771–775. doi: 10.1080/09553008914552031. [DOI] [PubMed] [Google Scholar]
- Ziboh V. A., Miller A. M., Yunis A. A., Jimenez J. J., Kursunoglu I. Arachidonic acid metabolism by rat chloroleukemia cells in culture. Cancer Res. 1981 Jan;41(1):12–17. [PubMed] [Google Scholar]



