Abstract
In mammals, Ca2+ and 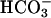 ions play a critical role in the regulation of sperm function, most likely by regulation of cAMP levels. Mammalian germ cells contain a soluble adenylyl cyclase (sAC) with properties distinct from the well characterized membrane-bound enzymes Here we investigated whether the cyclase expressed in mature spermatozoa has the properties of sAC and whether it is regulated by Ca2+. In addition to an
ions play a critical role in the regulation of sperm function, most likely by regulation of cAMP levels. Mammalian germ cells contain a soluble adenylyl cyclase (sAC) with properties distinct from the well characterized membrane-bound enzymes Here we investigated whether the cyclase expressed in mature spermatozoa has the properties of sAC and whether it is regulated by Ca2+. In addition to an 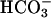 -dependent activation, the cyclase endogenous to human spermatozoa is stimulated 2- to 3-fold by Ca2+ in a concentration-dependent manner (EC50 ≈ 400 nM). In a similar fashion, Ca2+ activates the recombinant rat and human full-length sAC with similar EC50 values. The Ca2+ stimulation was also observed when sAC was activated with
-dependent activation, the cyclase endogenous to human spermatozoa is stimulated 2- to 3-fold by Ca2+ in a concentration-dependent manner (EC50 ≈ 400 nM). In a similar fashion, Ca2+ activates the recombinant rat and human full-length sAC with similar EC50 values. The Ca2+ stimulation was also observed when sAC was activated with 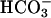 , was independent of calmodulin, and was associated with an increase in Vmax without changes in Km for ATP-Mg2+. An increase in intracellular Ca2+ by ionophore or by a muscarinic cholinergic receptor agonist increases cAMP in cells transfected with FL-hsAC, but not in mock-transfected cells. Similarly, both Ca2+ and
, was independent of calmodulin, and was associated with an increase in Vmax without changes in Km for ATP-Mg2+. An increase in intracellular Ca2+ by ionophore or by a muscarinic cholinergic receptor agonist increases cAMP in cells transfected with FL-hsAC, but not in mock-transfected cells. Similarly, both Ca2+ and 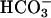 stimulate cAMP accumulation in human spermatozoa. These findings provide evidence that human spermatozoa express a cyclase with the properties of sAC and that Ca2+ can substitute for
stimulate cAMP accumulation in human spermatozoa. These findings provide evidence that human spermatozoa express a cyclase with the properties of sAC and that Ca2+ can substitute for 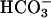 in the stimulation of this enzyme, underscoring an important role for sAC in the control of sperm functions.
in the stimulation of this enzyme, underscoring an important role for sAC in the control of sperm functions.
When released from the seminiferous epithelium, mammalian spermatozoa are immotile and unable to fertilize an egg. Acquisition of competence for fertilization requires a number of maturational processes, including activation of motility in the epididymis and capacitation in the female reproductive tract. This latter phenomenon coincides with the onset of hyperactivated motility, and the ability to undergo acrosome reaction (AR) in response to ZP3, a component of the egg extracellular matrix. All these processes require extracellular Ca2+ (1–4). Although the exact mechanisms regulating Ca2+ entry into the spermatozoon are not completely understood, it is generally accepted that an influx of Ca2+ is critical for these maturational events (5–7). Several channels that contribute to the control of membrane permeability to ions in the sperm membrane have been identified. These include voltage-gated calcium channels such as N-, R-, and T-type calcium channels (8, 9), cyclic nucleotide-gated channels (10, 11), and transient receptor potential channels (12, 13). Recently, two voltage-gated channel genes (CatSper and CatSper2) have been shown to be expressed exclusively in mammalian male germ cells, and immunofluorescence studies show a localization in the principal piece of the flagellum (14, 15). Although currents associated with CatSper and CatSper2 could not be studied in a reconstitution system, these channels appear to be essential for fertilization as the genetic ablation of CatSper causes male infertility.
One of the mechanisms by which Ca2+ is involved in spermatozoon function is through regulation of intracellular cAMP. This cyclic nucleotide has been shown to promote both capacitation and AR (16–18), and studies in different species demonstrated that an increase in Ca2+ induces an increase in sperm cAMP, or that Ca2+ is required for cAMP accumulation induced by 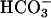 and progesterone, which are essential for capacitation and AR (3, 18, 19). The site of integration between these signaling pathways may be at several levels. In guinea pig sperm, it has been suggested that
and progesterone, which are essential for capacitation and AR (3, 18, 19). The site of integration between these signaling pathways may be at several levels. In guinea pig sperm, it has been suggested that 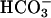 is required for Ca2+ uptake at the membrane level (6). Further studies (3, 20, 21) have pointed to the possibility that Ca2+ and
is required for Ca2+ uptake at the membrane level (6). Further studies (3, 20, 21) have pointed to the possibility that Ca2+ and 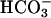 regulate the adenylyl cyclase (AC) present in spermatozoa, even though conflicting data have been reported.
regulate the adenylyl cyclase (AC) present in spermatozoa, even though conflicting data have been reported.
The properties of the AC present in mammalian spermatozoa remain a matter of debate. Although some studies suggest that a cyclase regulated by G proteins may be activated by the zona pellucida ZP3 (22), several reports have indicated the presence of a distinct form of cyclase not coupled to G proteins (23–26). The presence of more than one cyclase in sperm is possible in view of the data available on the AC present in maturing spermatids. In addition to the membrane-bound G protein-regulated ACIII (27, 28), differentiating germ cells contain an unconventional form of AC, soluble AC (sAC), with properties clearly distinct from the well characterized membrane-bound AC (25, 26, 29, 30). sAC has no identifiable transmembrane domain, is not regulated by forskolin or guanine nucleotide, but is stimulated by 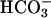 ions, and is structurally related to cyclases found in prokaryotes (31). Consistent with the retention of such a soluble cyclase in sperm, it has been shown that mammalian spermatozoa express an AC that is sensitive to
ions, and is structurally related to cyclases found in prokaryotes (31). Consistent with the retention of such a soluble cyclase in sperm, it has been shown that mammalian spermatozoa express an AC that is sensitive to 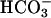 anions (6, 32, 33). Through activation of AC and an increase in cAMP,
anions (6, 32, 33). Through activation of AC and an increase in cAMP, 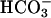 induces capacitation and capacitation-associated hyperactivated motility, which are all required for the penetration of the egg (4, 34–36).
induces capacitation and capacitation-associated hyperactivated motility, which are all required for the penetration of the egg (4, 34–36).
To elucidate the molecular basis of the interaction between Ca2+ and cAMP signaling in the control of sperm function, we have tested the hypothesis that sAC is sensitive to both Ca2+ and 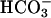 , and therefore this enzyme is a site for the integration of the two pathways. Our data demonstrate that indeed sAC is regulated by Ca2+ at physiological concentrations both in vitro and in intact cells.
, and therefore this enzyme is a site for the integration of the two pathways. Our data demonstrate that indeed sAC is regulated by Ca2+ at physiological concentrations both in vitro and in intact cells.
Materials and Methods
Culture Media and Reagents. All culture media were obtained from GIBCO/BRL. Restriction enzymes and ligases were from Roche Molecular Biochemicals. Human total testis RNA was obtained from Clontech and Biochain Institute (Hayward, CA). The [α-32]ATP and [3H]cAMP were from Perkin–Elmer NEN (Boston), and 125I-cAMP 2′-O-succinyl-3-[125I]iodotyrosine methyl ester from Amersham Pharmacia. Ultrapure (>99.99%) CaCl2, MgCl2, MnCl2, and NaHCO3 were purchased from Aldrich. Unless specified, all chemicals were of the purest grade available from Sigma.
Spermatozoa Preparations. Freshly ejaculated semen samples were obtained from patients at the Reproductive Endocrinology and Infertility laboratory at the Stanford Medical Center Gynecology Clinic. After semen analysis, normal semen samples (sperm concentration >40 × 106/ml, >50% progressive motility, and >60% normal morphology) were processed according to the methods described (29). For AC assay, the washed spermatozoa were resuspended in lysis buffer (50 mM Tris·HCl, pH 7.5/1 mM DTT/10 μg/ml leupeptin/10 μg/ml soybean trypsin inhibitor/0.7 mg/ml pepstatin A/50 mM benzamidine/1 mM PMSF) and sonicated four times for 30 sec each (Branson Sonifier 450) at a power setting of 6 with 30-sec ice-cooling periods. Sonicated sperm samples were first centrifuged at 14,000 × g for 20 min, and the supernatants were centrifuged again at 100,000 × g for 20 min at 4°C, to obtain a cytosolic fraction. Pellets (14,000 × g) were resuspended in lysis buffer to obtain the particulate fraction.
RT-PCR and cDNA Cloning. For the amplification of full-length human sAC (FL-hsAC), human testis total RNA (5 μg) was reverse transcribed with Moloney murine leukemia virus RT, by using oligo(dT) primer for first-strand cDNA synthesis (First-Strand complementary DNA synthesis kit, Pharmacia Biotech, Piscataway, NJ) according to manufacturer's protocols. PCR was performed directly from 3 μl of first-strand cDNA by using 500 nmol each of hsAC-1f (5′-TGGAGACTGGATCCTGTCAC-3′) and hsAC-1r (5′-GATAATCACAAGGACATAGTGCT-3′) primers, and 2.5 units of Hercules polymerase (Stratagene). The amplified FL-hsAC fragment of correct size was cloned in-frame with the V5 epitope of the pcDNA3.1/V5-His/TOPO mammalian expression vector by using a Eukaryotic TOPO TA cloning kit (Invitrogen) following manufacturer's directions and sequenced to verify the absence of mutations. Rat FL-sAC was cloned as mentioned (29).
Generation and Purification of sAC Antibodies. Three rabbit polyclonal antisera were used. One antiserum (mid-peptide) was against a synthetic peptide of 22 amino acids with the sequence NH2-CKHYKERQTNLQNRVKTLLDDK-COOH. This sequence corresponds to amino acids 571–592 of rat sAC, a region located after the C1C2 catalytic domain. Two other antisera were raised against a fusion protein of 469 amino acids of the N terminus of rat sAC (amino acids 1–469) and 210 amino acids of the C terminus of rat sAC (amino acids 1399–1608) fused to GST (GST-rsAC). The sera from the fourth boost were affinity-purified with the peptide or the GST-rsAC fusion protein immobilized on activated CH Sepharose 4B column. Selectivity and specificity of the antisera for the rsAC were confirmed by Western blotting and ELISA (data not shown). These antibodies crossreacted with lesser affinity with hsAC.
Immunoprecipitation. The cytosolic extracts of recombinant V5-tagged FL-hsAC and native sAC of human sperm were immunoprecipitated by using the V5 and sAC antibody (peptide or N terminus and C terminus) respectively. Extracts were incubated either with preimmune or immune serum immobilized to beads overnight at 4°C by gentle mixing. Samples were then centrifuged at 1000 × g for 3 min. The pellets were washed three times with TBS-B (20 mM Tris·HCl and 150 mM NaCl, pH 7.6, containing 0.5 mg/ml BSA), resuspended in a similar volume of TBS-B, and an aliquot was used for the AC assay.
Transient Transfection of HEK293 Cells. Expression constructs were transiently transfected into HEK293 cells by the calcium phosphate method. Briefly, HEK293 cells (2 × 106 cells) were seeded in 10-cm dishes and grown in DMEM containing 10% FBS at 70% confluence. Twenty μg of DNA of each construct (for AC assay), 4 μg of pcDNA3.1 empty vector, or FL-hsAC-V5 construct (for cAMP assay) was transfected to HEK293 by CaCl2 precipitation (125 mM) in 2-[bis (2-hydroxyehtyl)-amino] ethane sulfonic acid. For AC assay, cells were harvested 24 h after transfection with lysis buffer and disrupted by homogenization in a Dounce homogenizer by 20 strokes. Cell homogenates were centrifuged first at 14,000 × g for 20 min at 4°C. Supernatants were centrifuged again at 100,000 × g for 20 min at 4°C to obtain the cytosolic fraction.
Adenylyl Cyclase Assay. In vitro AC assay was performed as described by Alvarez and Daniels (37) with some modification (29). AC activity was measured on the enzyme prepared either from cells expressing an empty plasmid and different sAC constructs or from the washed human sperm preparation as described (29). Free Ca2+ concentrations were generated from a series of CaCl2 solutions buffered with 200 μM EGTA in the assay calculated by using the webmaxc V2.10 version of the maxchelator program.
Cyclic AMP Accumulation. Cellular cAMP accumulation was measured and analyzed essentially as described (38). Empty vector, FL-hsAC-V5 plasmid DNA-transfected HEK293 cells were washed with Ca2+- and Mg2+-free PBS 24 h after transfection, and starved for 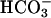 by incubating them for 6 h at 37°C in
by incubating them for 6 h at 37°C in 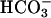 -free DMEM (buffered with 25 mM Hepes, pH 7.4, instead of NaHCO3), followed by 15-min incubation at 37°C in DMEM-I (DMEM containing 500 μM phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine) with or without 25 mM
-free DMEM (buffered with 25 mM Hepes, pH 7.4, instead of NaHCO3), followed by 15-min incubation at 37°C in DMEM-I (DMEM containing 500 μM phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine) with or without 25 mM 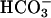 , pH 7.4. To study the effect of Ca2+, cells were incubated for 15 min at 37°C with 10 μM Ca2+ ionophore A23187, 10 μM ionomycin, or increasing concentrations (from 10 nM to 100 μM) of carbachol. To measure cAMP level, washed human spermatozoa (2–5 × 106 cells) were incubated at 37°C in DMEM-I with or without 25 mM
, pH 7.4. To study the effect of Ca2+, cells were incubated for 15 min at 37°C with 10 μM Ca2+ ionophore A23187, 10 μM ionomycin, or increasing concentrations (from 10 nM to 100 μM) of carbachol. To measure cAMP level, washed human spermatozoa (2–5 × 106 cells) were incubated at 37°C in DMEM-I with or without 25 mM 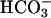 , pH 7.4, and treated with 10 μM A23187 for 15 min. At the end of the incubation period, reaction was stopped and cAMP was extracted as described (29). cAMP accumulation was then measured by an RIA (39) after acetylating the samples (29). TCA-precipitated protein pellets of transfected HEK293 cells were dissolved in 1× sample buffer without 2-mercaptoethanol, [62.5 mM Tris·HCl, pH 6.8/10% glycerol/2% (wt/vol) SDS/0.0025% (wt/vol) bromophenol blue] for protein assay and Western blot analysis.
, pH 7.4, and treated with 10 μM A23187 for 15 min. At the end of the incubation period, reaction was stopped and cAMP was extracted as described (29). cAMP accumulation was then measured by an RIA (39) after acetylating the samples (29). TCA-precipitated protein pellets of transfected HEK293 cells were dissolved in 1× sample buffer without 2-mercaptoethanol, [62.5 mM Tris·HCl, pH 6.8/10% glycerol/2% (wt/vol) SDS/0.0025% (wt/vol) bromophenol blue] for protein assay and Western blot analysis.
The protein concentration of the TCA-precipitated protein samples was measured according to the method of Bradford (40) by using a DC protein assay kit (Bio-Rad).
Western Blotting. Protein pellets from TCA precipitates were fractionated by electrophoresis on SDS/8% polyacrylamide gels. Proteins were then transferred onto an Immobilon membrane (Millipore), and Western blot analysis was performed (29) with the V5 antibody (Invitrogen).
Statistical Analysis. When reported, the mean ± SEM, Km, P, and EC50 values were calculated by using prism 2.01 software (Graph-Pad, San Diego). Km for the ATP was calculated after omitting the data point showing substrate inhibition. P values were evaluated by using two-tailed paired t test.
Results
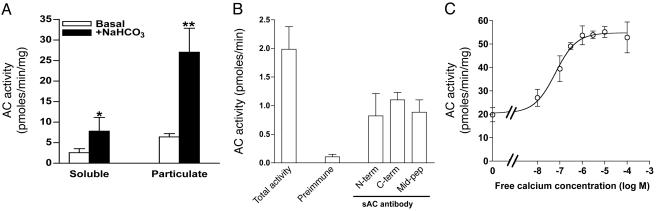
Ca2+ Regulation of Native AC Activity from Human Spermatozoa. To determine whether sAC is expressed in human spermatozoa, sperm homogenates were fractionated into soluble and particulate components by ultracentrifugation. AC activity recovered in both fractions was stimulated between 2.5- and 6-fold by 50 mM 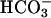 (Fig. 1A). Antibodies raised against three distinct epitopes of rat sAC immunoprecipitated the soluble (Fig. 1B) or solubilized (data not shown) sperm cyclase activity. Forskolin failed to stimulate the sperm AC activity recovered in soluble or particulate fractions (data not shown), excluding the presence in these preparations of Ca2+-regulated conventional membrane-bound cyclases such as ACI, ACIII, or ACVIII. These immunological and biochemical properties indicate that sAC is expressed in human spermatozoa.
(Fig. 1A). Antibodies raised against three distinct epitopes of rat sAC immunoprecipitated the soluble (Fig. 1B) or solubilized (data not shown) sperm cyclase activity. Forskolin failed to stimulate the sperm AC activity recovered in soluble or particulate fractions (data not shown), excluding the presence in these preparations of Ca2+-regulated conventional membrane-bound cyclases such as ACI, ACIII, or ACVIII. These immunological and biochemical properties indicate that sAC is expressed in human spermatozoa.
Fig. 1.
Detection of sAC activity in human sperm and the effect of Ca2+ on sAC activity. (A) AC activity in the soluble and particulate fraction of human spermatozoa was measured in the presence of 5 mM MgCl2 with or without 25 mM 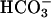 . The data reported are mean ± SEM of five independent experiments with different semen samples. *, P < 0.05; **, P < 0.001. (B) Immunoprecipitation of AC activity of human spermatozoa using N terminus (N-term), C terminus (C-term), or mid-peptide (mid-pep) anti-sAC antibodies. The AC activity was measured in the immunoprecipitation pellets in the presence of 5 mM MnCl2 as described in Materials and Methods. The data shown are mean ± SEM of four separate experiments. (C) AC activity was determined in the presence of 5 mM MgCl2 at the indicated concentration of free Ca2+ in the cytosolic fractions of washed human spermatozoa. The data represent mean ± SEM of a triplicate determination of an experiment that was repeated at least five times with different human sperm samples with similar results.
. The data reported are mean ± SEM of five independent experiments with different semen samples. *, P < 0.05; **, P < 0.001. (B) Immunoprecipitation of AC activity of human spermatozoa using N terminus (N-term), C terminus (C-term), or mid-peptide (mid-pep) anti-sAC antibodies. The AC activity was measured in the immunoprecipitation pellets in the presence of 5 mM MnCl2 as described in Materials and Methods. The data shown are mean ± SEM of four separate experiments. (C) AC activity was determined in the presence of 5 mM MgCl2 at the indicated concentration of free Ca2+ in the cytosolic fractions of washed human spermatozoa. The data represent mean ± SEM of a triplicate determination of an experiment that was repeated at least five times with different human sperm samples with similar results.
When human sperm soluble extracts were incubated with free Ca2+ concentrations ranging between 10–8 and 10–4 M, a concentration-dependent increase (2- to 3-fold) in cyclase activity was observed (Fig. 1C). In the five experiments performed, the calculated EC50 for Ca2+ was 393 ± 140 nM. A 2- to 3-fold stimulation of AC activity by Ca2+ was also detected when sperm homogenates were used (data not shown). However, not all human sperm preparations behaved identically. In two of the eight sperm extracts studied, a clear Ca2+ dose-dependent activation in sperm homogenate could not be constructed. No clear correlation between sperm quality and Ca2+ stimulation of sperm AC could be established.
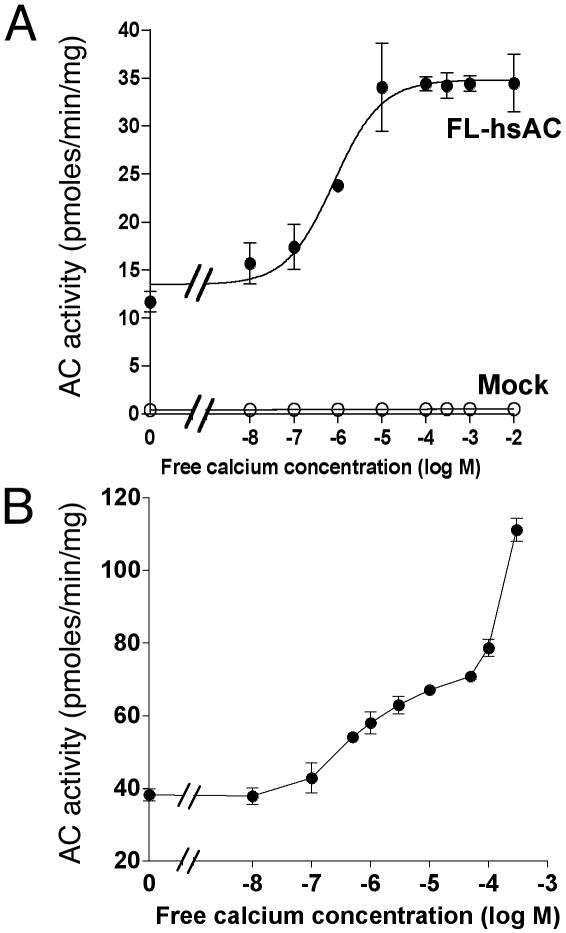
Ca2+ Regulation of the Recombinant hsAC and Rat sAC. To verify whether sAC is the target for Ca2+ activation observed in sperm extracts, recombinant FL-hsAC and rat FL-sAC was used to investigate the effects of Ca2+. Cells transfected with the empty vector (mock) did not show any significant AC activity in the absence or presence of Ca2+ (Fig. 2A). Conversely, significant AC activity was detected in the 100,000 × g supernatant of the FL-sAC-transfected cells, and increasing free Ca2+ in the assay caused a concentration-dependent increase in AC activity (Fig. 2). Identical results were obtained with the recombinant rat FL-sAC (see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). In the five preparations studied, the EC50 values for Ca2+ stimulation were 327 ± 200 nM, a figure similar to that observed with the AC endogenous to spermatozoa. The effect of Ca2+ was not detectable when recombinant FL-sAC or endogenous sAC from human spermatozoa was assayed in the presence of 5 mM Mn2+ (data not shown), rather than Mg2+.
Fig. 2.
Stimulation of recombinant sAC by Ca2+. AC activity was measured in the cytosolic fractions prepared from HEK293 cells transfected with either empty vector or FL-hsAC plasmid DNA in the presence of 5 mM MgCl2 at the indicated concentration of free Ca2+ without (A) or with (B)25 mM 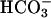 . The data represent mean ± SEM of triplicate determination of at least four experiments performed.
. The data represent mean ± SEM of triplicate determination of at least four experiments performed.
Previous studies indicated that Ca2+ and 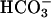 have synergistic effects on cAMP accumulation in intact spermatozoa (6, 19). Because both Ca2+ and
have synergistic effects on cAMP accumulation in intact spermatozoa (6, 19). Because both Ca2+ and 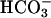 activate sAC, a possible additive or synergistic effect on sAC activation was investigated. In the presence of 25 mM
activate sAC, a possible additive or synergistic effect on sAC activation was investigated. In the presence of 25 mM 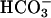 , Ca2+ activated sAC in a biphasic manner with a high-affinity component (EC50 = ≈300 nM), followed by a further not saturable stimulation at concentrations of >100 μM Ca2+ (Fig. 2B). Similar effects were observed with a truncated C1C2-hsAC (see Fig. 7, which is published as supporting information on the PNAS web site).
, Ca2+ activated sAC in a biphasic manner with a high-affinity component (EC50 = ≈300 nM), followed by a further not saturable stimulation at concentrations of >100 μM Ca2+ (Fig. 2B). Similar effects were observed with a truncated C1C2-hsAC (see Fig. 7, which is published as supporting information on the PNAS web site).
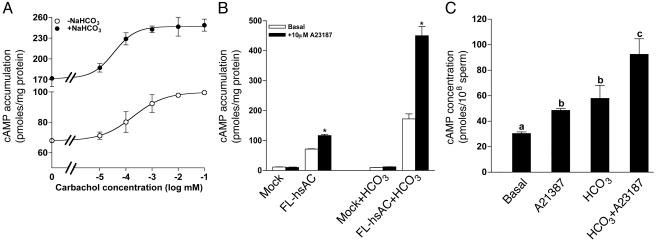
Effect of Ca2+ on the cAMP Level of Intact Cells Expressing FL-hsAC. To determine whether Ca2+ also activates sAC in intact cells, the V5-tagged FL-hsAC construct was expressed in HEK293 cells and cAMP levels were measured after stimulation with either the Ca2+ ionophore A23187, which produces a Ca2+ influx from extracellular medium, or with the muscarinic cholinergic receptor agonist carbachol, which recruits Ca2+ from intracellular stores. HEK293 cells have been shown to express functional muscarinic cholinergic receptors coupled to Ca2+ mobilization (41, 42). As shown in Fig. 3, cells expressing the recombinant FL-hsAC display an increase in cAMP levels when compared with cells transfected with an empty vector (mock). Treatment with either A23187 or carbachol caused an ≈2-fold increase in cAMP accumulation in FL-hsAC-transfected cells, but not in mock-transfected cells (Fig. 3). Similar results were observed with a different Ca2+ ionophore, ionomycin (data not shown). The increase in the cAMP level in cells expressing recombinant FL-sAC enzyme after A23187 or carbachol stimulation is not due to the difference in the sAC expression because the immunoreactivity of FL-hsAC was comparable in all groups (Fig. 3 Upper). The whole of these data therefore provide evidence that sAC is activated by Ca2+ and that cells expressing the FL-sAC acquire the ability to accumulate cAMP in response to an increase in intracellular Ca2+.
Fig. 3.
An increase in intracellular Ca2+ stimulates cAMP accumulation in FL-hsAC-transfected HEK293 cells. HEK293 cells transfected with an empty vector or vector containing FL-hsAC-V5 were challenged for an additional 15 min with DMEM-I (basal), DMEM-I and 10 μM A23187, or DMEM-I and 10 μM carbachol. cAMP was extracted and measured by an RIA as described in Materials and Methods. *, P < 0.05 compared with basal cAMP levels. (Upper) Western blot analysis of the TCA precipitates from HEK293 cells transfected with an empty vector (mock, M) or with FL-hsAC-V5 (FL) plasmid DNA. Extracts were fractionated by electrophoresis on SDS/8% PAGE and probed with an anti-V5 antibody. A, A23187; C, carbachol. The data represent three different experiments repeated with similar results.
Consistent with cell-free cyclase data, treatment of FL-hsAC-transfected HEK293 cells with 25 mM 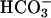 caused a 2- to 3-fold increase in cAMP levels (Fig. 4). Treatment of these cells with carbachol further increased the cAMP level in a dose-dependent manner, both in the absence and presence of
caused a 2- to 3-fold increase in cAMP levels (Fig. 4). Treatment of these cells with carbachol further increased the cAMP level in a dose-dependent manner, both in the absence and presence of 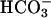 (Fig. 4A). An additive or synergistic effect of
(Fig. 4A). An additive or synergistic effect of 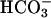 and Ca2+ was also observed with A23187 (Fig. 4B).
and Ca2+ was also observed with A23187 (Fig. 4B).
Fig. 4.
cAMP accumulation in sAC-expressing cells and human sperm after an increase in intracellular Ca2+ and 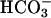 . (A) HEK293 cells were transfected with empty vector or vector containing FL-hsAC-V5. Transfected cells were challenged for 15 min with an increasing concentration of carbachol in the absence or presence of 25 mM
. (A) HEK293 cells were transfected with empty vector or vector containing FL-hsAC-V5. Transfected cells were challenged for 15 min with an increasing concentration of carbachol in the absence or presence of 25 mM 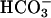 .(B) Empty vector (mock) and FL-hsAC-transfected HEK293 cells were incubated with DMEM-I with or without 25 mM
.(B) Empty vector (mock) and FL-hsAC-transfected HEK293 cells were incubated with DMEM-I with or without 25 mM 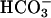 and treated with 10 μM A23187. *, P < 0.05 compared with basal cAMP levels. (C) Washed human sperm (2–5 × 106 cells) were incubated at 37°C in DMEM-I (basal) or DMEM-I and 25 mM
and treated with 10 μM A23187. *, P < 0.05 compared with basal cAMP levels. (C) Washed human sperm (2–5 × 106 cells) were incubated at 37°C in DMEM-I (basal) or DMEM-I and 25 mM 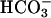 with or without 10 μM A23187 for 15 min. Different letters denote a significant difference between treatments (P < 0.05). The data represent the mean ± SEM of a triplicate determination of three separate experiments.
with or without 10 μM A23187 for 15 min. Different letters denote a significant difference between treatments (P < 0.05). The data represent the mean ± SEM of a triplicate determination of three separate experiments.
To verify whether Ca2+ and 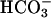 have a similar effect on endogenous sAC, washed sperm were treated with 10 μM A23187 and 25 mM
have a similar effect on endogenous sAC, washed sperm were treated with 10 μM A23187 and 25 mM 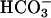 . Either A23187 or
. Either A23187 or 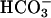 caused a significant increase in cAMP from the basal level (Fig. 4C). In a manner similar to the recombinant FL-hsAC expressed in HEK293 cells, the effect of Ca2+ and
caused a significant increase in cAMP from the basal level (Fig. 4C). In a manner similar to the recombinant FL-hsAC expressed in HEK293 cells, the effect of Ca2+ and 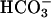 on the endogenous cAMP level of human sperm was additive/synergistic with up to a 4-fold increase in cAMP.
on the endogenous cAMP level of human sperm was additive/synergistic with up to a 4-fold increase in cAMP.
Mechanism of Ca2+ Stimulation of sAC. The calmodulin (CaM) antagonist trifluoperazine and W7 inhibited Ca2+ stimulation of sAC at concentrations of 100 μM or higher (data not shown). Because this inhibition by CaM antagonist was detected only at high concentrations, the involvement of CaM in Ca2+ stimulation of sAC was investigated further. The 100,000 × g supernatant of FL-hsAC-transfected cells was treated with Ca2+ and CaM. Whereas Ca2+ stimulates FL-hsAC activity ≈2-fold, CaM failed to stimulate this AC activity further (see Fig. 8A, which is published as supporting information on the PNAS web site). Similarly, immunoprecipitation of V5-tagged FL-hsAC with anti-V5 antibody did not recover an immunoreactive CaM in the pellet, as assessed by SDS/PAGE and Western blot (Fig. 8B). When these CaM-depleted sAC preparations were used for measuring cyclase activity, 10 μM Ca2+ still stimulated sAC activity 2-fold, and addition of CaM (10 μM) again failed to further stimulate sAC activity (data not shown). In a converse experiment, CaM antibody did not immunoprecipitate significant amounts of sAC activity, and CaM depletion by immunoprecipitation with anti-CaM antibody did not affect sAC activation by Ca2+ in the depleted supernatants (data not shown).
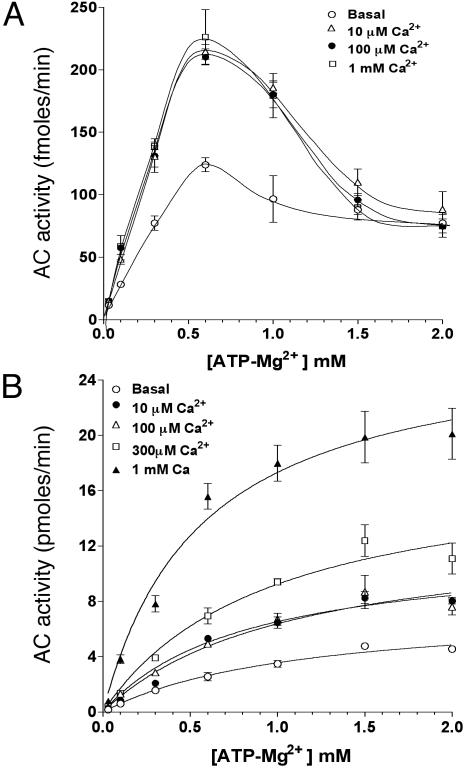
When sAC proteins with progressive deletions of the C terminus were used for Ca2+ activation, they were all stimulated by nanomolar Ca2+ including the minimal catalytic construct C1-C2 (see Fig. 9, which is published as supporting information on the PNAS web site), indicating that the site of Ca2+ interaction with the enzyme is close to the catalytic center. In view of this proximity with the ATP-binding site, we evaluated the effect of Ca2+ on the kinetic properties of sAC partially purified by immunoprecipitation. At concentrations up to 1 mM, Ca2+ produced an increase in sAC Vmax (fmol/min; basal = 329 ± 69; 10 μM Ca2+ = 633 ± 100) without altering the Km for the substrate. The biphasic effect of ATP-Mg2+ on sAC activity is probably due to substrate inhibition, as recently reported (43); however, under these conditions, Ca2+ addition did not modify the inhibitory effects of ATP-Mg2+ (Fig. 5A). Consistent with our previous finding (Fig. 2), when Ca2+ effects were measured in the presence of 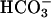 , a biphasic effect was evident (Fig. 5B). Concentrations of 10 or 100 μM Ca2+ increased the Vmax of the cyclase without affecting the Km for the substrate (Km in mM: basal = 1.12 ± 0.2; 10 μM Ca2+ = 1.07 ± 0.2). Higher Ca2+ concentrations further stimulated the activity, but in this latter case, a decrease in Km for ATP-Mg2+ was observed (Km: 1 mM Ca2+ = 0.56 ± 0.13). The inhibitory effects of ATP at concentrations of 1 mM or higher were not detectable in the presence of
, a biphasic effect was evident (Fig. 5B). Concentrations of 10 or 100 μM Ca2+ increased the Vmax of the cyclase without affecting the Km for the substrate (Km in mM: basal = 1.12 ± 0.2; 10 μM Ca2+ = 1.07 ± 0.2). Higher Ca2+ concentrations further stimulated the activity, but in this latter case, a decrease in Km for ATP-Mg2+ was observed (Km: 1 mM Ca2+ = 0.56 ± 0.13). The inhibitory effects of ATP at concentrations of 1 mM or higher were not detectable in the presence of 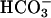 (Fig. 5B).
(Fig. 5B).
Fig. 5.
Kinetic properties of Ca2+-activated sAC. Soluble extract of FL-hsAC-V5-transfected HEK293 cells were immunoprecipitated with V5 antibody, and AC activity was measured in the presence of 10 mM MgCl2, 200 μM EGTA, and increasing concentrations of free Ca2+ as a function of substrate ATP-Mg2+. sAC kinetics were assessed at the indicated concentration of free Ca2+ in the absence (A) or presence (B) of 25 mM 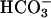 . The data represent mean ± SEM of triplicate determination of at least two experiments.
. The data represent mean ± SEM of triplicate determination of at least two experiments.
Discussion
Countless studies have shown that Ca2+, 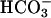 , and cAMP play critical roles in mammalian fertilization by regulating sperm motility, capacitation, capacitation-associated hyperactivated motility, and the induction of AR (44–47). However, the mechanistic details of the interactions between these signals were not known. Here we present evidence that sAC is active in human spermatozoa and is a sensor for both
, and cAMP play critical roles in mammalian fertilization by regulating sperm motility, capacitation, capacitation-associated hyperactivated motility, and the induction of AR (44–47). However, the mechanistic details of the interactions between these signals were not known. Here we present evidence that sAC is active in human spermatozoa and is a sensor for both 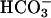 and Ca2+. Thus, sAC is a point of convergence of the two signals and undoubtedly plays a critical role in the positive feedback that controls sperm function.
and Ca2+. Thus, sAC is a point of convergence of the two signals and undoubtedly plays a critical role in the positive feedback that controls sperm function.
The properties of AC activity recovered in human spermatozoa and those of recombinant hsAC clearly overlap. To exclude possible contamination of membrane-bound cyclase, a high-speed soluble fraction from sperm extract was used for most of the studies. Although only 20–30% of the total AC was recovered in this fraction, the cyclase properties resemble that of the sperm homogenate. The soluble sperm cyclase was recognized by three antibodies raised against the N terminus, the C terminus, and a central epitope of the recombinant sAC. Furthermore, the sperm AC activity is stimulated by 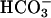 , and had low affinity for ATP; all properties shared with recombinant sAC. Finally, two peaks of activity were recovered from gel filtration chromatography of the sperm extract, a finding similar to our previous observations in rat and other species (29, 48, 49). The first peak of activity comigrated with FL-sAC. The second peak may reflect the presence of a splicing variant and/or processing of the FL-sAC as we have shown in rat (29). Thus, the activity recovered in the soluble extract of human spermatozoa likely corresponds to sAC, and may share the particulate activity released during the preparation of the extract. Even though it was difficult to extract sperm particulate activity, the properties of this activity also overlap with those of sAC, being activated by
, and had low affinity for ATP; all properties shared with recombinant sAC. Finally, two peaks of activity were recovered from gel filtration chromatography of the sperm extract, a finding similar to our previous observations in rat and other species (29, 48, 49). The first peak of activity comigrated with FL-sAC. The second peak may reflect the presence of a splicing variant and/or processing of the FL-sAC as we have shown in rat (29). Thus, the activity recovered in the soluble extract of human spermatozoa likely corresponds to sAC, and may share the particulate activity released during the preparation of the extract. Even though it was difficult to extract sperm particulate activity, the properties of this activity also overlap with those of sAC, being activated by 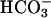 and Ca2+. However, we cannot exclude that additional cyclases are present in the plasma membrane or acrosome of spermatozoa. Forskolin-stimulated activity could not be detected in human spermatozoa, suggesting that conventional cyclases are a minor portion of the overall sperm cAMP-generating capacity.
and Ca2+. However, we cannot exclude that additional cyclases are present in the plasma membrane or acrosome of spermatozoa. Forskolin-stimulated activity could not be detected in human spermatozoa, suggesting that conventional cyclases are a minor portion of the overall sperm cAMP-generating capacity.
Our data demonstrate that Ca2+ stimulates both endogenous and recombinant sAC. Ca2+ activates sAC by binding to a saturable site with an affinity in the nanomolar range. Occupancy of this site produces an increase in Vmax of the enzyme without changes in the Km for the ATP-Mg2+ substrate. This site has little or no affinity for Mg2+ because we detected effects of nanomolar Ca2+ in the presence of 10 mM Mg2+. Because a similar high-affinity Ca2+ binding site was detected in the truncated C1-C2, we propose that this metal-binding site is in, or in the vicinity of, the catalytic center. The high-affinity binding site is most likely not the consequence of CaM binding to sAC. No CaM was detected in the immunoprecipitates of sAC, and CaM did not affect the Ca2+ stimulation in all conditions tested. However, we cannot exclude that a different Ca2+-binding protein mediates the Ca2+ effects by binding close to the catalytic region of sAC. Although CaM inhibitors blocked Ca2+ stimulation, the concentration required is ≈10-fold higher than the EC50 for binding to CaM (50, 51).
There is a second mode of interaction of Ca2+ with the enzyme (43). This nonsaturable, low-affinity interaction is of uncertain physiological significance in view of the mM Ca2+ concentrations required. This second binding site may be the consequence of Ca2+ competition with Mg2+ at one of the two metal-binding sites in the catalytic center of the enzyme, as defined by the 3D structure (52). This substitution of Mg2+ with Ca2+ may produce a change in Km for the substrate. However, because this low-affinity interaction cannot be detected in FL-sAC in the absence of 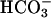 , we should entertain the possibility that
, we should entertain the possibility that 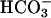 binding impacts the affinity of one of the catalytic metal-ion-binding sites, or stabilizes Ca2+ binding in other pockets of the catalytic domain. A possible mechanism of
binding impacts the affinity of one of the catalytic metal-ion-binding sites, or stabilizes Ca2+ binding in other pockets of the catalytic domain. A possible mechanism of 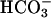 interaction with the catalytic center of cyanobacteria cyclase has been reported recently (53).
interaction with the catalytic center of cyanobacteria cyclase has been reported recently (53).
Although some studies could not detect a change in Ca2+ (47), it is accepted that an increase in Ca2+ in sperm occurs during capacitation, as well as during the AR. The intracellular Ca2+ level increases from 25 ± 10 to 160 ± 40 nM in bovine sperm (54), and from 30 to 200 nM in human sperm (55), when spermatozoa are exposed to capacitation medium. During the AR, intracellular Ca2+ increases rapidly and may reach a concentration up to 10 μM (9, 51, 56). Therefore, Ca2+ changes that occur during capacitation and AR are well within the range of the sensitivity of sAC to Ca2+, as we demonstrated by using both the endogenous sAC, as well as the recombinant FL-sAC expressed in heterologous systems. Thus, an influx in Ca2+ during the AR should produce a 2- to 3-fold increase in sAC activity that synergizes with  . Whether changes in the concentration of
. Whether changes in the concentration of 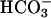 in spermatozoa occur during capacitation is at present unknown. However, it is well established that ejaculated spermatozoa are exposed to 10–30 mM of
in spermatozoa occur during capacitation is at present unknown. However, it is well established that ejaculated spermatozoa are exposed to 10–30 mM of 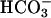 in the seminal and reproductive tract fluid (57–59).
in the seminal and reproductive tract fluid (57–59).
The presence of sAC in spermatozoa and its regulation by Ca2+ suggest that a positive feedback loop may be activated during AR, and perhaps earlier during capacitation. According to this hypothesis, changes in membrane composition and/or physical properties in the spermatozoon, as well as changes in membrane potential, cause an increase in Ca2+ and perhaps a change in 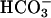 concentration. The increase in Ca2+ and
concentration. The increase in Ca2+ and 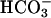 ion concentration causes an increase in sAC activity, and the ensuing increase in cAMP produces further increases in Ca2+ influx through activation of cyclic nucleotide-gated channels and the CatSper Ca2+ channel, as well as through PKA- or perhaps cAMP GEF-mediated processes. Through this positive feedback loop, the sustained increase of cAMP levels due to sAC activation by Ca2+ and
ion concentration causes an increase in sAC activity, and the ensuing increase in cAMP produces further increases in Ca2+ influx through activation of cyclic nucleotide-gated channels and the CatSper Ca2+ channel, as well as through PKA- or perhaps cAMP GEF-mediated processes. Through this positive feedback loop, the sustained increase of cAMP levels due to sAC activation by Ca2+ and 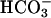 is likely to be involved in the functional changes required for fertilization. The observation that male sterility of CatSper–/– is associated with an absence of cAMP- or cGMP-mediated Ca2+ influx indicates that the cAMP-mediated signaling machinery is an important component for controlling Ca2+ permeability (14, 15). Positive feedback loops are known to produce switch-like effects and irreversible biological transitions, properties that fit well with the characteristics of capacitation and AR (60).
is likely to be involved in the functional changes required for fertilization. The observation that male sterility of CatSper–/– is associated with an absence of cAMP- or cGMP-mediated Ca2+ influx indicates that the cAMP-mediated signaling machinery is an important component for controlling Ca2+ permeability (14, 15). Positive feedback loops are known to produce switch-like effects and irreversible biological transitions, properties that fit well with the characteristics of capacitation and AR (60).
In summary, we report here that Ca2+ is a physiological regulator of sAC in addition to 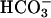 . This Ca2+ regulation opens the possibility that changes in membrane ion permeability in the spermatozoa are coupled to changes in cAMP through activation of sAC. Based on these considerations and the data indicating an important role of Ca2+ and
. This Ca2+ regulation opens the possibility that changes in membrane ion permeability in the spermatozoa are coupled to changes in cAMP through activation of sAC. Based on these considerations and the data indicating an important role of Ca2+ and 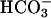 in mammalian sperm functions in fertilization, we hypothesize that inactivating mutation(s) in sAC will produce a disruption of sperm function and infertility.
in mammalian sperm functions in fertilization, we hypothesize that inactivating mutation(s) in sAC will produce a disruption of sperm function and infertility.
Supplementary Material
Acknowledgments
We thank Fang Xie for assistance in the generation and characterization of N terminus and C terminus sAC antibodies; Linda Lan for technical assistance; and Caren Spencer for editing. This work was supported by National Institutes of Health Grant HD 31544 (to M.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AC, adenylyl cyclase; sAC, soluble AC; FL-sAC, full-length sAC; FL-hsAC, full-length human sAC; AR, acrosome reaction; CaM, calmodulin.
Data deposition: The cDNA sequence of human sAC has been deposited in the GenBank database (accession no. AF271058).
References
- 1.DasGupta, S., Mills, C. L. & Fraser, L. R. (1993) J. Reprod. Fertil. 99, 135–143. [DOI] [PubMed] [Google Scholar]
- 2.Handrow, R. R., First, N. L. & Parrish, J. J. (1989) J. Exp. Zool. 252, 174–182. [DOI] [PubMed] [Google Scholar]
- 3.Hyne, R. V. & Garbers, D. L. (1979) Proc. Natl. Acad. Sci. USA 76, 5699–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visconti, P. E., Bailey, J. L., Moore, G. D., Pan, D., Olds-Clarke, P. & Kopf, G. S. (1995) Development (Cambridge, U.K.) 121, 1129–1137. [DOI] [PubMed] [Google Scholar]
- 5.Visconti, P. E., Stewart-Savage, J., Blasco, A., Battaglia, L., Miranda, P., Kopf, G. S. & Tezon, J. G. (1999) Biol. Reprod. 61, 76–84. [DOI] [PubMed] [Google Scholar]
- 6.Garbers, D. L., Tubb, D. J. & Hyne, R. V. (1982) J. Biol. Chem. 257, 8980–8984. [PubMed] [Google Scholar]
- 7.Garde, J. & Roldan, E. R. (2000) J. Reprod. Fertil. 118, 57–68. [DOI] [PubMed] [Google Scholar]
- 8.Wennemuth, G., Westenbroek, R. E., Xu, T., Hille, B. & Babcock, D. F. (2000) J. Biol. Chem. 275, 21210–21217. [DOI] [PubMed] [Google Scholar]
- 9.Arnoult, C., Kazam, I. G., Visconti, P. E., Kopf, G. S., Villaz, M. & Florman, H. M. (1999) Proc. Natl. Acad. Sci. USA 96, 6757–6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weyand, I., Godde, M., Frings, S., Weiner, J., Muller, F., Altenhofen, W., Hatt, H. & Kaupp, U. B. (1994) Nature 368, 859–863. [DOI] [PubMed] [Google Scholar]
- 11.Serrano, C. J., Trevino, C. L., Felix, R. & Darszon, A. (1999) FEBS Lett. 462, 171–176. [DOI] [PubMed] [Google Scholar]
- 12.Westenbroek, R. E. & Babcock, D. F. (1999) Dev. Biol. 207, 457–469. [DOI] [PubMed] [Google Scholar]
- 13.Jungnickel, M. K., Marrero, H., Birnbaumer, L., Lemos, J. R. & Florman, H. M. (2001) Nat. Cell Biol. 3, 499–502. [DOI] [PubMed] [Google Scholar]
- 14.Quill, T. A., Ren, D., Clapham, D. E. & Garbers, D. L. (2001) Proc. Natl. Acad. Sci. USA 98, 12527–12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren, D., Navarro, B., Perez, G., Jackson, A. C., Hsu, S., Shi, Q., Tilly, J. L. & Clapham, D. E. (2001) Nature 413, 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visconti, P. E., Moore, G. D., Bailey, J. L., Leclerc, P., Connors, S. A., Pan, D., Olds-Clarke, P. & Kopf, G. S. (1995) Development (Cambridge, U.K.) 121, 1139–1150. [DOI] [PubMed] [Google Scholar]
- 17.Parrish, J. J., Susko-Parrish, J. L., Uguz, C. & First, N. L. (1994) Biol. Reprod. 51, 1099–1108. [DOI] [PubMed] [Google Scholar]
- 18.Parinaud, J. & Milhet, P. (1996) J. Clin. Endocrinol. Metab. 81, 1357–1360. [DOI] [PubMed] [Google Scholar]
- 19.Rojas, F. J., Patrizio, P., Do, J., Silber, S., Asch, R. H. & Moretti-Rojas, I. (1993) Endocrinology 133, 3030–3033. [DOI] [PubMed] [Google Scholar]
- 20.Rojas, F. J., Bruzzone, M. E. & Moretti-Rojas, I. (1992) Hum. Reprod. 7, 1131–1135. [DOI] [PubMed] [Google Scholar]
- 21.Hyne, R. V. & Garbers, D. L. (1979) Biol. Reprod. 21, 1135–1142. [DOI] [PubMed] [Google Scholar]
- 22.Leclerc, P. & Kopf, G. S. (1995) Biol. Reprod. 52, 1227–1233. [DOI] [PubMed] [Google Scholar]
- 23.Rojas, F. J. & Bruzzone, M. E. (1992) Hum. Reprod. 7, 1126–1130. [DOI] [PubMed] [Google Scholar]
- 24.Forte, L. R., Bylund, D. B. & Zahler, W. L. (1983) Mol. Pharmacol. 24, 42–47. [PubMed] [Google Scholar]
- 25.Adamo, S., Conti, M., Geremia, R. & Monesi, V. (1980) Biochem. Biophys. Res. Commun. 97, 607–613. [DOI] [PubMed] [Google Scholar]
- 26.Braun, T. & Dods, R. F. (1975) Proc. Natl. Acad. Sci. USA 72, 1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautier-Courteille, C., Salanova, M. & Conti, M. (1998) Endocrinology 139, 2588–2599. [DOI] [PubMed] [Google Scholar]
- 28.Defer, N., Marinx, O., Poyard, M., Lienard, M. O., Jegou, B. & Hanoune, J. (1998) FEBS Lett. 424, 216–220. [DOI] [PubMed] [Google Scholar]
- 29.Jaiswal, B. S. & Conti, M. (2001) J. Biol. Chem. 276, 31698–31708. [DOI] [PubMed] [Google Scholar]
- 30.Sinclair, M. L., Wang, X. Y., Mattia, M., Conti, M., Buck, J., Wolgemuth, D. J. & Levin, L. R. (2000) Mol. Reprod. Dev. 56, 6–11. [DOI] [PubMed] [Google Scholar]
- 31.Buck, J., Sinclair, M. L., Schapal, L., Cann, M. J. & Levin, L. R. (1999) Proc. Natl. Acad. Sci. USA 96, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamura, N., Tajima, Y., Soejima, A., Masuda, H. & Sugita, Y. (1985) J. Biol. Chem. 260, 9699–9705. [PubMed] [Google Scholar]
- 33.Garty, N. B. & Salomon, Y. (1987) FEBS Lett. 218, 148–152. [DOI] [PubMed] [Google Scholar]
- 34.Shi, Q. X. & Roldan, E. R. (1995) Biol. Reprod. 52, 540–546. [DOI] [PubMed] [Google Scholar]
- 35.Jaiswal, B. S. & Majumder, G. C. (1996) Int. J. Androl. 19, 97–102. [DOI] [PubMed] [Google Scholar]
- 36.Boatman, D. E. & Robbins, R. S. (1991) Biol. Reprod. 44, 806–813. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez, R. & Daniels, D. V. (1992) Anal. Biochem. 203, 76–82. [DOI] [PubMed] [Google Scholar]
- 38.Oki, N., Takahashi, S. I., Hidaka, H. & Conti, M. (2000) J. Biol. Chem. 275, 10831–10837. [DOI] [PubMed] [Google Scholar]
- 39.Steiner, A. L., Pagliara, A. S., Chase, L. R. & Kipnis, D. M. (1972) J. Biol. Chem. 247, 1114–1120. [PubMed] [Google Scholar]
- 40.Bradford, M. M. (1976) Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 41.Chiono, M., Mahey, R., Tate, G. & Cooper, D. M. (1995) J. Biol. Chem. 270, 1149–1155. [DOI] [PubMed] [Google Scholar]
- 42.Fagan, K. A., Mahey, R. & Cooper, D. M. (1996) J. Biol. Chem. 271, 12438–12444. [DOI] [PubMed] [Google Scholar]
- 43.Litvin, T. N., Kamenetsky, M., Zarifyan, A., Buck, J. & Levin, L. R. (2003) J. Biol. Chem. 278, 15922–15926. [DOI] [PubMed] [Google Scholar]
- 44.Jaiswal, B. S. & Eisenbach, M. (2001) in Fertilization, ed. Hardy, D. M. (Academic, San Diego), pp. 57–117.
- 45.Breitbart, H. (2002) J. Reprod. Immunol. 53, 151–159. [DOI] [PubMed] [Google Scholar]
- 46.Visconti, P. E., Westbrook, V. A., Chertihin, O., Demarco, I., Sleight, S. & Diekman, A. B. (2002) J. Reprod. Immunol. 53, 133–150. [DOI] [PubMed] [Google Scholar]
- 47.Yanagimachi, R. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. (Raven, New York), pp. 189–317.
- 48.Gordeladze, J. O., Abyholm, T., Cusan, L., Clausen, O. P. & Hansson, V. (1982) Arch. Androl. 8, 199–204. [DOI] [PubMed] [Google Scholar]
- 49.Braun, T. (1991) Methods Enzymol. 195, 130–136. [DOI] [PubMed] [Google Scholar]
- 50.Massom, L., Lee, H. & Jarrett, H. W. (1990) Biochemistry 29, 671–681. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Gonzalez, I., De La Vega-Beltran, J. L., Santi, C. M., Florman, H. M., Felix, R. & Darszon, A. (2001) Dev. Biol. 236, 210–219. [DOI] [PubMed] [Google Scholar]
- 52.Tesmer, J. J., Sunahara, R. K., Johnson, R. A., Gosselin, G., Gilman, A. G. & Sprang, S. R. (1999) Science 285, 756–760. [DOI] [PubMed] [Google Scholar]
- 53.Cann, M. J., Hammer, A., Zhou, J. & Kanacher, T. (2003) J. Biol. Chem., in press. [DOI] [PubMed]
- 54.Dragileva, E., Rubinstein, S. & Breitbart, H. (1999) Biol. Reprod. 61, 1226–1234. [DOI] [PubMed] [Google Scholar]
- 55.Baldi, E., Casano, R., Falsetti, C., Krausz, C., Maggi, M. & Forti, G. (1991) J. Androl. 12, 323–330. [PubMed] [Google Scholar]
- 56.Bailey, J. L. & Storey, B. T. (1994) Mol. Reprod. Dev. 39, 297–308. [DOI] [PubMed] [Google Scholar]
- 57.Brooks, D. E. (1983) Aust. J. Biol. Sci. 36, 205–221. [DOI] [PubMed] [Google Scholar]
- 58.David, A., Brackett, B. G., Garcia, C. R. & Mastroianni, L., Jr. (1969) J. Reprod. Fertil. 19, 285–289. [DOI] [PubMed] [Google Scholar]
- 59.Murdoch, R. N. & White, I. G. (1968) Aust. J. Biol. Sci. 21, 961–972. [DOI] [PubMed] [Google Scholar]
- 60.Ferrell, J. E., Jr. (2002) Curr. Opin. Cell Biol. 14, 140–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.