Abstract
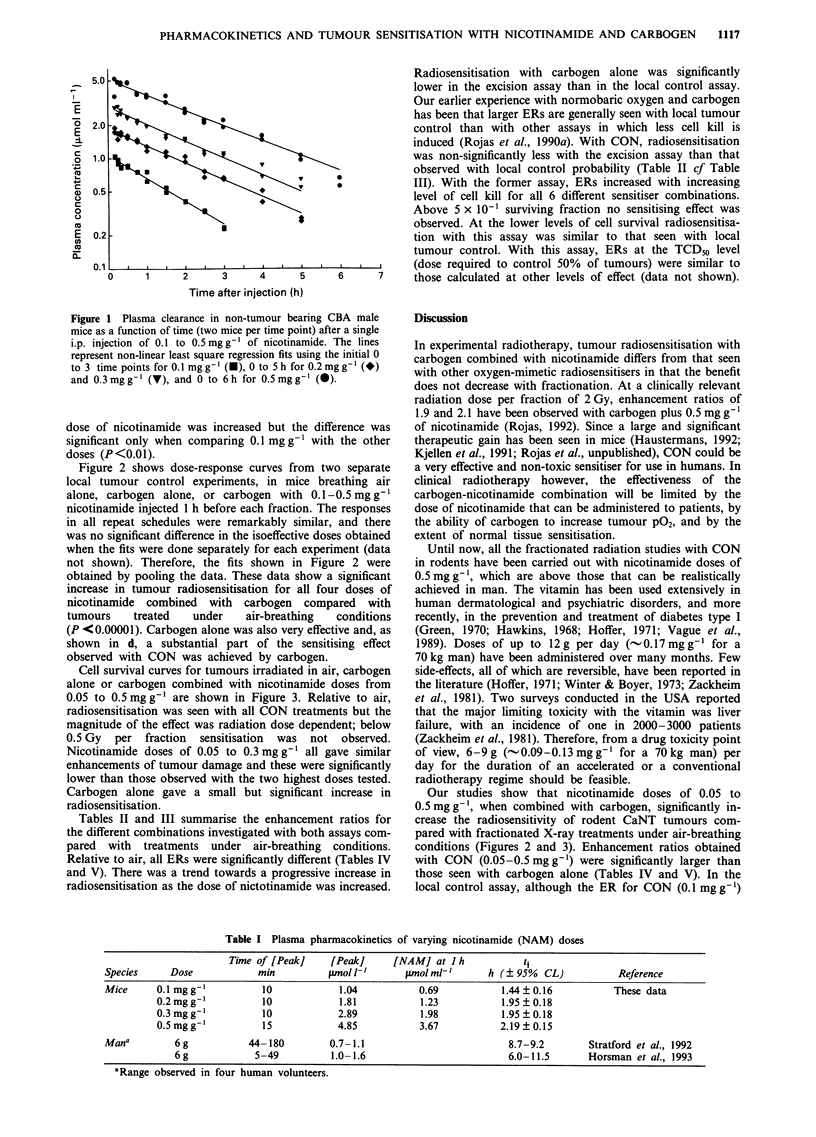
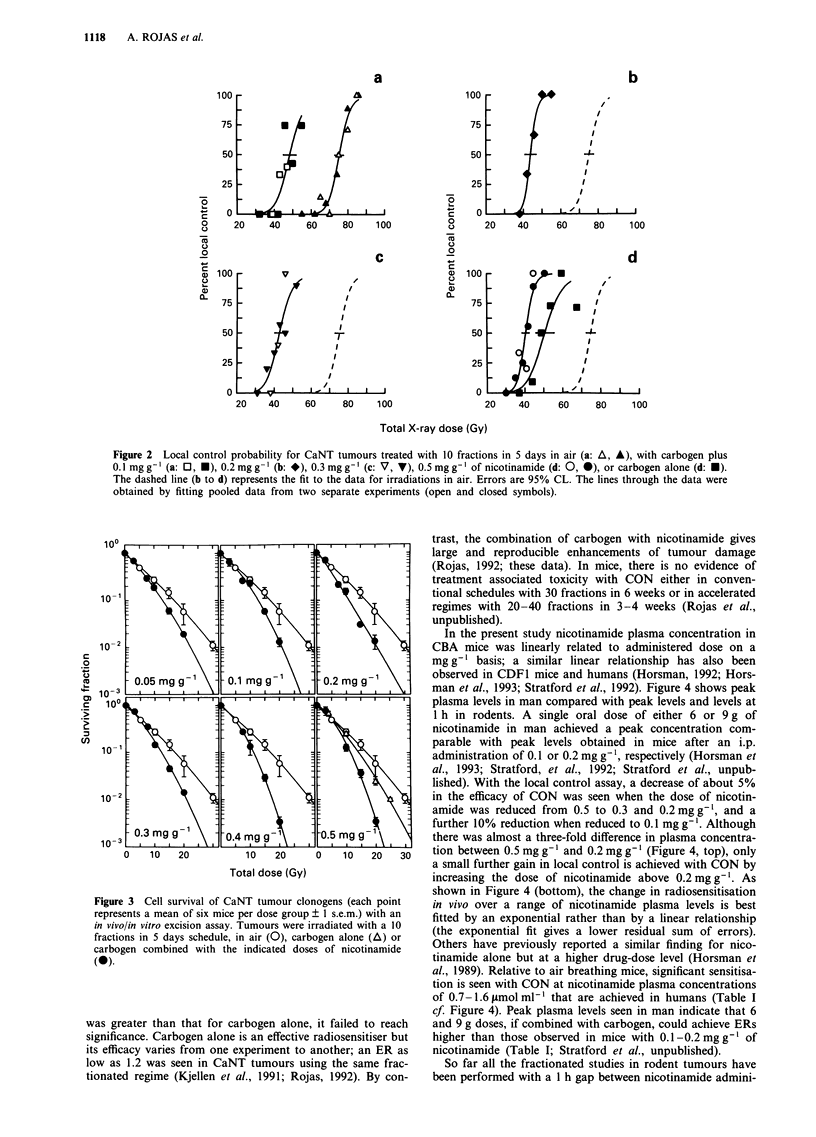
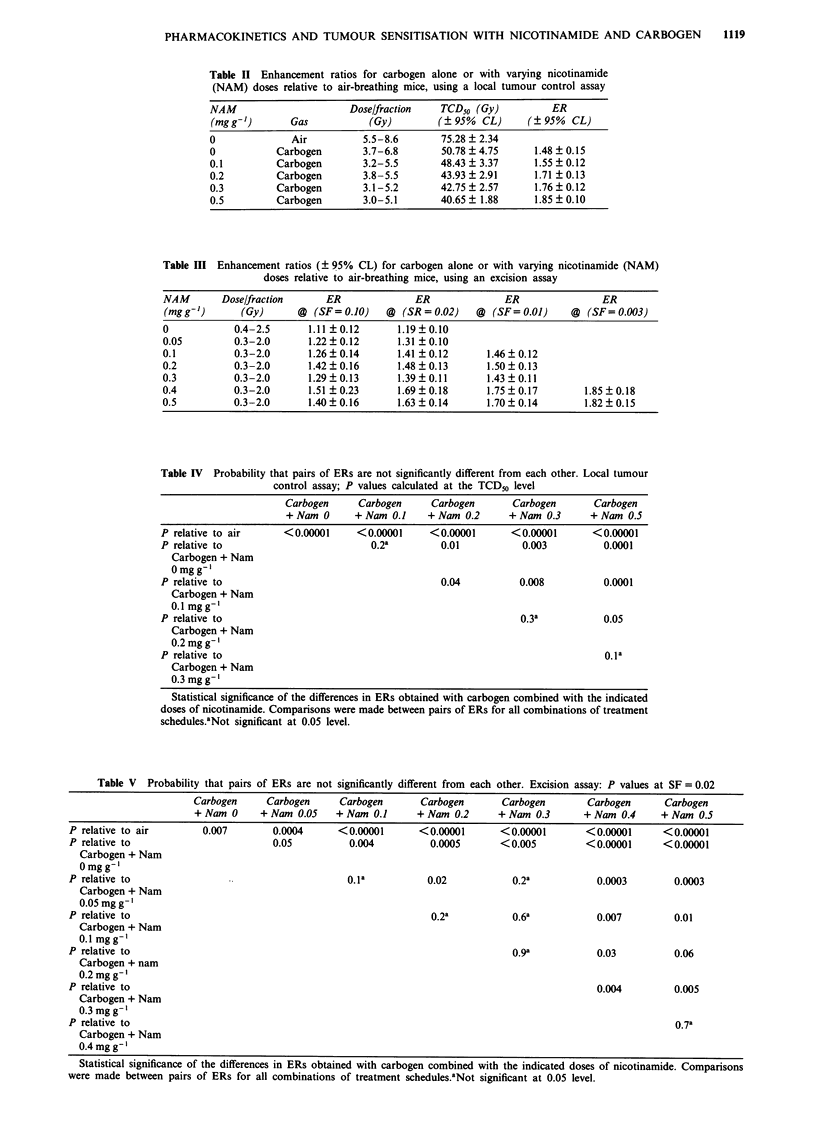
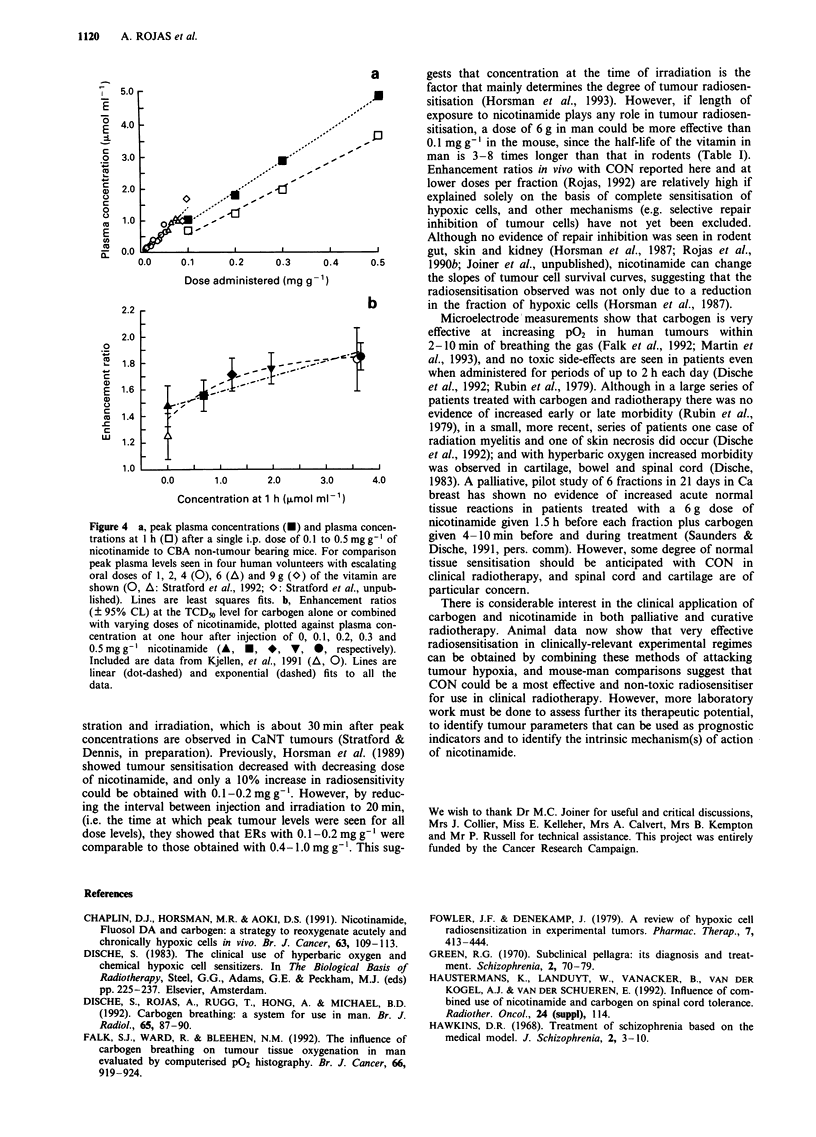
Plasma concentrations, after administration of varying doses of nicotinamide, were measured in CBA male mice using a newly-developed high performance liquid chromatography assay. In all dose groups, peak levels were observed within the first 15 min after an i.p. administration of 0.1, 0.2, 0.3 or 0.5 mg g-1 of nicotinamide. There was a clear dose-dependent increase in plasma concentration with increasing dose, with almost a five-fold lower concentration (1.0 vs 4.9 mumol ml-1) achieved with a dose of 0.1 mg g-1 compared with 0.5 mg g-1, respectively. The half-life of nicotinamide increased from 1.4 h to 2.2 h over the dose range (P < 0.01). Comparisons with previous pharmacokinetic data in humans show that clinically-relevant oral doses of 6 and 9 g in humans give plasma levels slightly higher than those achieved at 1 h with doses of 0.1 to 0.2 mg g-1 in mice. Tumour radiosensitisation with carbogen alone, and with carbogen combined with varying doses of nicotinamide (0.05 to 0.5 mg g-1), was investigated using a 10-fraction in 5 days X-ray schedule. Relative to air-breathing mice, a statistically significant increase in sensitisation was observed with both a local tumour control and with an in vivo/in vitro excision assay (P < or = 0.007). With the local control assay, a trend was observed towards lower enhancement ratios (ERs) with decreasing nicotinamide dose (from 1.85 to 1.55); carbogen alone was almost as effective as when combined with 0.1 mg g-1 of nicotinamide. With the excision assay, ERs for carbogen combined with nicotinamide increased with decreased levels of cell survival. At a surviving fraction of 0.02, enhancement ratios of 1.39-1.48 were obtained for carbogen plus 0.1 to 0.3 mg g-1 of nicotinamide. These were lower than those seen with the two higher doses of 0.4 to 0.5 mg g-1 (ERs = 1.63-1.69).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaplin D. J., Horsman M. R., Aoki D. S. Nicotinamide, Fluosol DA and Carbogen: a strategy to reoxygenate acutely and chronically hypoxic cells in vivo. Br J Cancer. 1991 Jan;63(1):109–113. doi: 10.1038/bjc.1991.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham J. W. Implantation of the tongue and floor of mouth--what factors really do contribute to necrosis? Radiother Oncol. 1992 May;24(1):64–66. doi: 10.1016/0167-8140(92)90356-y. [DOI] [PubMed] [Google Scholar]
- Dische S., Rojas A., Rugg T., Hong A., Michael B. D. Carbogen breathing: a system for use in man. Br J Radiol. 1992 Jan;65(769):87–90. doi: 10.1259/0007-1285-65-769-87. [DOI] [PubMed] [Google Scholar]
- Falk S. J., Ward R., Bleehen N. M. The influence of carbogen breathing on tumour tissue oxygenation in man evaluated by computerised p02 histography. Br J Cancer. 1992 Nov;66(5):919–924. doi: 10.1038/bjc.1992.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J. F., Denekamp J. A review of hypoxic cell radiosensitization in experimental tumors. Pharmacol Ther. 1979;7(3):413–444. doi: 10.1016/0163-7258(79)90039-1. [DOI] [PubMed] [Google Scholar]
- Hatlevoll R., Hagen S., Hansen H. S., Hultborn R., Jakobsen A., Mäntylä M., Modig H., Munck-Wikland E., Nygaard K., Rosengren B. Bleomycin/cis-platin as neoadjuvant chemotherapy before radical radiotherapy in localized, inoperable carcinoma of the esophagus. A prospective randomized multicentre study: the second Scandinavian trial in esophageal cancer. Radiother Oncol. 1992 Jun;24(2):114–116. doi: 10.1016/0167-8140(92)90288-6. [DOI] [PubMed] [Google Scholar]
- Hendry J. H. Quantitation of the radiotherapeutic importance of naturally-hypoxic normal tissues from collated experiments with rodents using single doses. Int J Radiat Oncol Biol Phys. 1979 Jul;5(7):971–976. doi: 10.1016/0360-3016(79)90602-3. [DOI] [PubMed] [Google Scholar]
- Hill R. P. Sensitizers and radiation dose fractionation: results and interpretations. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1049–1054. doi: 10.1016/0360-3016(86)90223-3. [DOI] [PubMed] [Google Scholar]
- Hoffer A. Megavitamin B-3 therapy for schizophrenia. Can Psychiatr Assoc J. 1971 Dec;16(6):499–504. doi: 10.1177/070674377101600605. [DOI] [PubMed] [Google Scholar]
- Horsman M. R. Carbogen and nicotinamide: expectations too high? (response to J. Martin Brown) Radiother Oncol. 1992 Jun;24(2):121–122. doi: 10.1016/0167-8140(92)90291-2. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Chaplin D. J., Brown J. M. Radiosensitization by nicotinamide in vivo: a greater enhancement of tumor damage compared to that of normal tissues. Radiat Res. 1987 Mar;109(3):479–489. [PubMed] [Google Scholar]
- Horsman M. R., Chaplin D. J., Brown J. M. Tumor radiosensitization by nicotinamide: a result of improved perfusion and oxygenation. Radiat Res. 1989 Apr;118(1):139–150. [PubMed] [Google Scholar]
- Horsman M. R., Høyer M., Honess D. J., Dennis I. F., Overgaard J. Nicotinamide pharmacokinetics in humans and mice: a comparative assessment and the implications for radiotherapy. Radiother Oncol. 1993 May;27(2):131–139. doi: 10.1016/0167-8140(93)90133-s. [DOI] [PubMed] [Google Scholar]
- Kjellen E., Joiner M. C., Collier J. M., Johns H., Rojas A. A therapeutic benefit from combining normobaric carbogen or oxygen with nicotinamide in fractionated X-ray treatments. Radiother Oncol. 1991 Oct;22(2):81–91. doi: 10.1016/0167-8140(91)90002-x. [DOI] [PubMed] [Google Scholar]
- Martin L., Lartigau E., Weeger P., Lambin P., Le Ridant A. M., Lusinchi A., Wibault P., Eschwege F., Luboinski B., Guichard M. Changes in the oxygenation of head and neck tumors during carbogen breathing. Radiother Oncol. 1993 May;27(2):123–130. doi: 10.1016/0167-8140(93)90132-r. [DOI] [PubMed] [Google Scholar]
- Overgaard J., Sand Hansen H., Lindeløv B., Overgaard M., Jørgensen K., Rasmusson B., Berthelsen A. Nimorazole as a hypoxic radiosensitizer in the treatment of supraglottic larynx and pharynx carcinoma. First report from the Danish Head and Neck Cancer Study (DAHANCA) protocol 5-85. Radiother Oncol. 1991;20 (Suppl 1):143–149. doi: 10.1016/0167-8140(91)90202-r. [DOI] [PubMed] [Google Scholar]
- Rojas A. ARCON: accelerated radiotherapy with carbogen and nicotinamide. BJR Suppl. 1992;24:174–178. [PubMed] [Google Scholar]
- Rojas A., Carl U., Reghebi K. Effect of normobaric oxygen on tumor radiosensitivity: fractionated studies. Int J Radiat Oncol Biol Phys. 1990 Mar;18(3):547–553. doi: 10.1016/0360-3016(90)90059-s. [DOI] [PubMed] [Google Scholar]
- Rubin P., Hanley J., Keys H. M., Marcial V., Brady L. Carbogen breathing during radiation therapy-the Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1979 Nov-Dec;5(11-12):1963–1970. doi: 10.1016/0360-3016(79)90946-5. [DOI] [PubMed] [Google Scholar]
- Stewart F. A., Denekamp J., Randhawa V. S. Skin sensitization by misonidazole: a demonstration of uniform mild hypoxia. Br J Cancer. 1982 Jun;45(6):869–877. doi: 10.1038/bjc.1982.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford M. R., Dennis M. F. High-performance liquid chromatographic determination of nicotinamide and its metabolites in human and murine plasma and urine. J Chromatogr. 1992 Nov 6;582(1-2):145–151. doi: 10.1016/0378-4347(92)80313-f. [DOI] [PubMed] [Google Scholar]
- Stratford M. R., Rojas A., Hall D. W., Dennis M. F., Dische S., Joiner M. C., Hodgkiss R. J. Pharmacokinetics of nicotinamide and its effect on blood pressure, pulse and body temperature in normal human volunteers. Radiother Oncol. 1992 Sep;25(1):37–42. doi: 10.1016/0167-8140(92)90193-x. [DOI] [PubMed] [Google Scholar]
- Winter S. L., Boyer J. L. Hepatic toxicity from large doses of vitamin B3 (nicotinamide). N Engl J Med. 1973 Nov 29;289(22):1180–1182. doi: 10.1056/NEJM197311292892208. [DOI] [PubMed] [Google Scholar]
- Zachkeim H. S., Vasily D. B., Westphal M. L., Hastings C. W. Reactions to niacinamide. J Am Acad Dermatol. 1981 Jun;4(6):736–738. doi: 10.1016/s0190-9622(81)80211-3. [DOI] [PubMed] [Google Scholar]



