Abstract
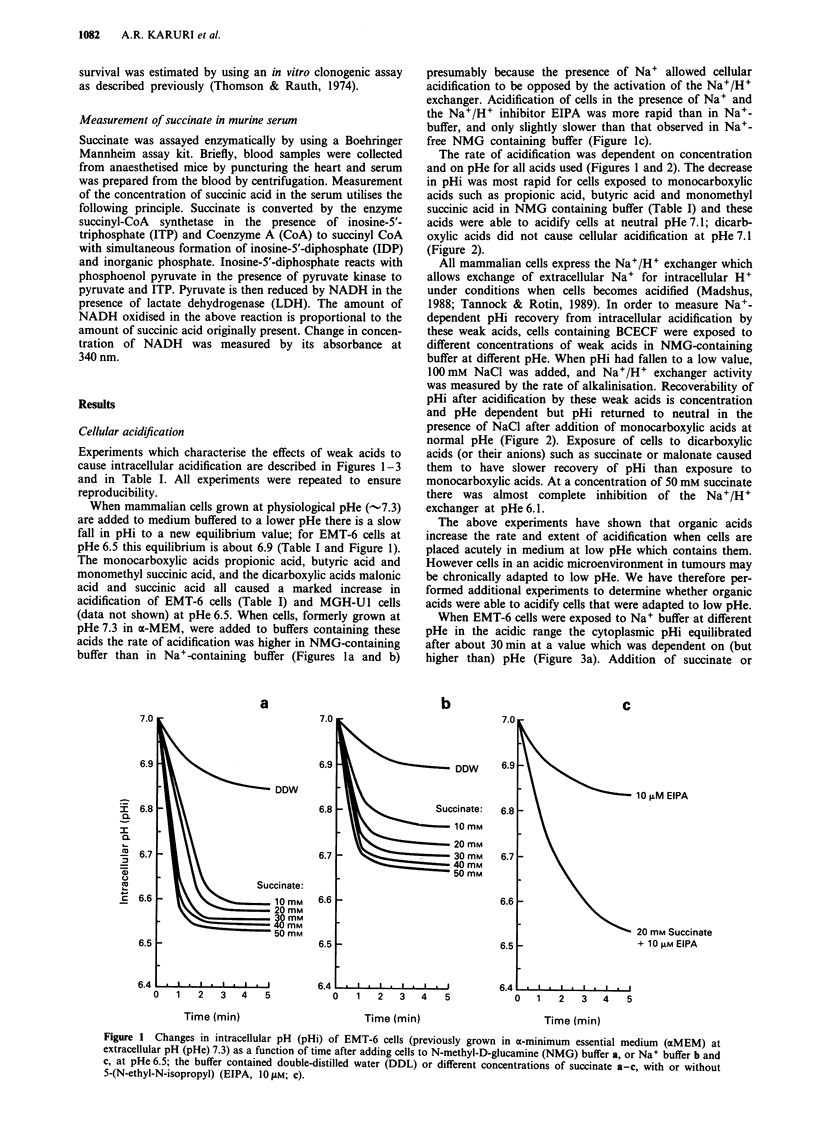
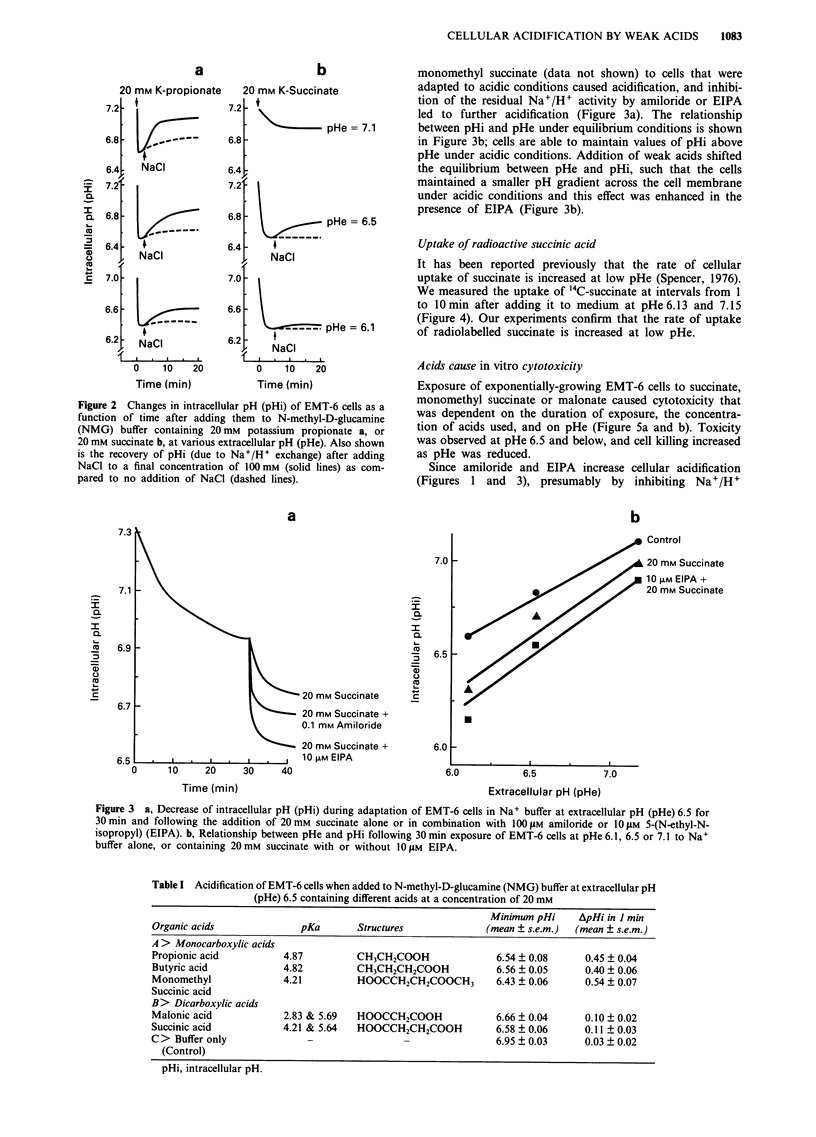
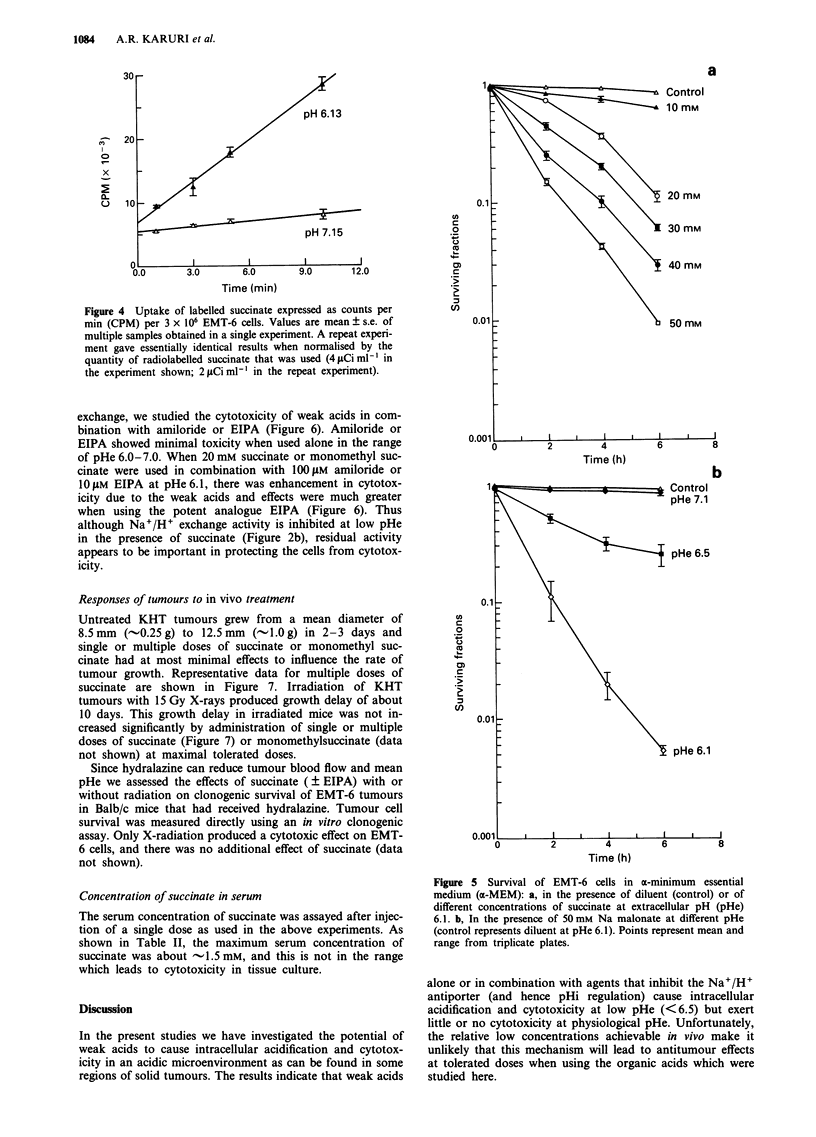
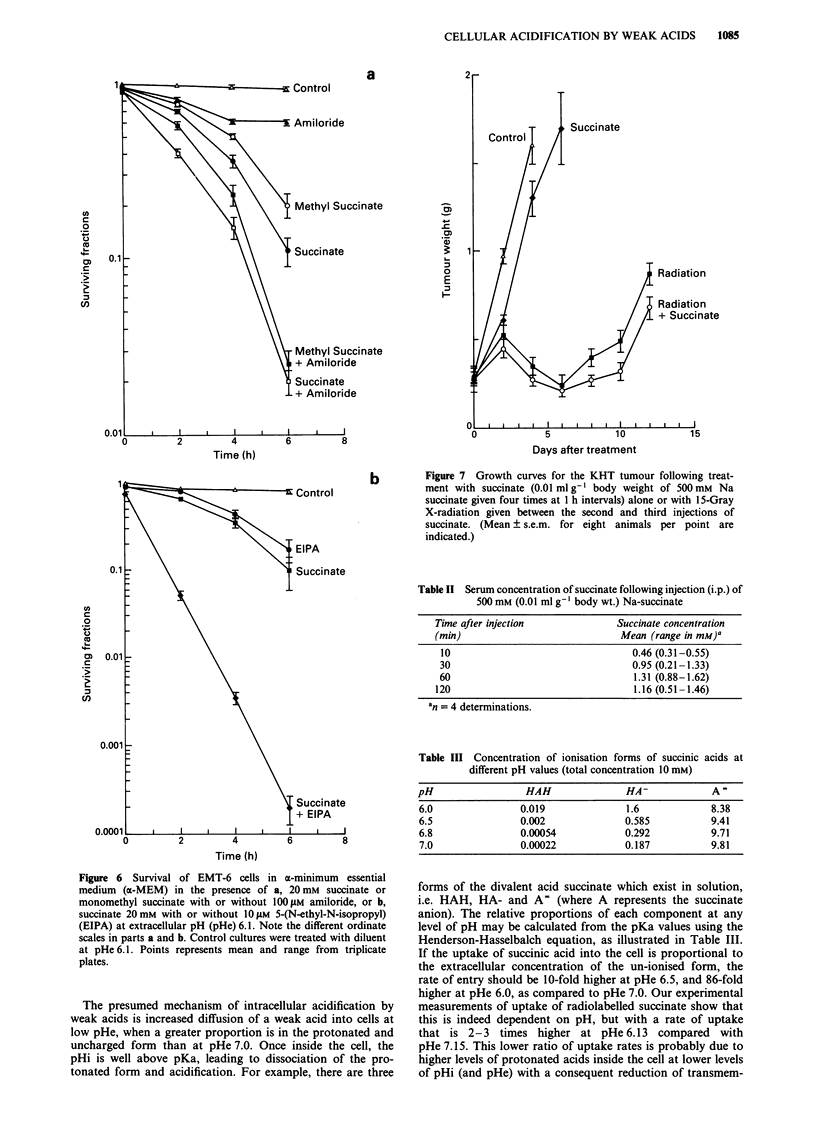
The mean extracellular pH (pHe) within solid tumours has been found to be lower than in normal tissues. Agents which cause intracellular acidification at low pHe might have selective toxicity towards cells in tumours. Weak acids (or their anions) with pKa values in the range of 4-6 have a higher proportion of molecules in the uncharged form at low pHe and can diffuse more rapidly into cells. The effects of organic acids including succinate, monomethyl succinate and malonate to acidify cells have been evaluated under conditions of different pHe in the acidic range. These weak acids caused intracellular acidification of murine EMT-6 and human MGH-U1 cells in a concentration and pHe dependent fashion. At concentrations of 10 mM and above, these acids also caused in vitro cytotoxicity to these cells at low pHe (< 6.5). The rate and extent of cellular acidification caused by these weak acids, and their cytotoxicity at low pHe, were enhanced by exposure to amiloride and 5-(N-ethyl-N-isopropyl)amiloride (EIPA), agents which inhibit Na+/H+ exchange, and hence the regulation of intracellular pH. Acid dependent cytotoxicity was also investigated in a murine solid tumour using the endpoints of growth delay and colony formation in vitro following treatment in vivo. Agents were tested alone or with 15 Gy X-rays to select a population of hypoxic (and presumably acidic) cells. Achievable serum concentrations of succinate were about 1 mM and no antitumour activity of succinate was detected when used in this way. It is concluded that weak acids are selectively taken up into cells, and can cause selective cellular acidification and toxicity, at low pHe in culture. Weak acids that are normal cellular metabolites are not toxic in vivo, but weak acids carrying cytotoxic groups offer the potential for selective uptake and toxicity under the conditions of low pHe that exist in many solid tumours.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer M. J., Tannock I. F. Regulation of intracellular pH in tumor cell lines: influence of microenvironmental conditions. Cancer Res. 1992 Aug 15;52(16):4441–4447. [PubMed] [Google Scholar]
- Deuticke B., Beyer E., Forst B. Discrimination of three parallel pathways of lactate transport in the human erythrocyte membrane by inhibitors and kinetic properties. Biochim Biophys Acta. 1982 Jan 4;684(1):96–110. doi: 10.1016/0005-2736(82)90053-0. [DOI] [PubMed] [Google Scholar]
- Dobrowsky E., Newell K., Tannock I. F. The potential of lactate and succinate to kill nutrient deprived tumor cells by intracellular acidification. Int J Radiat Oncol Biol Phys. 1991 Feb;20(2):275–279. doi: 10.1016/0360-3016(91)90104-c. [DOI] [PubMed] [Google Scholar]
- Jähde E., Glüsenkamp K. H., Klünder I., Hülser D. F., Tietze L. F., Rajewsky M. F. Hydrogen ion-mediated enhancement of cytotoxicity of bis-chloroethylating drugs in rat mammary carcinoma cells in vitro. Cancer Res. 1989 Jun 1;49(11):2965–2972. [PubMed] [Google Scholar]
- Lin J. C., Song C. W. Effects of hydralazine on the blood flow in RIF-1 tumors and normal tissues of mice. Radiat Res. 1990 Nov;124(2):171–177. [PubMed] [Google Scholar]
- Madshus I. H. Regulation of intracellular pH in eukaryotic cells. Biochem J. 1988 Feb 15;250(1):1–8. doi: 10.1042/bj2500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidorn R. P., Cragoe E. J., Jr, Tannock I. F. Therapeutic potential of analogues of amiloride: inhibition of the regulation of intracellular pH as a possible mechanism of tumour selective therapy. Br J Cancer. 1993 Feb;67(2):297–303. doi: 10.1038/bjc.1993.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen R. B., Wallach D. F. Transmembrane ion gradients and thermochemotherapy. Prog Clin Biol Res. 1982;107:103–107. [PubMed] [Google Scholar]
- Newell K. J., Tannock I. F. Reduction of intracellular pH as a possible mechanism for killing cells in acidic regions of solid tumors: effects of carbonylcyanide-3-chlorophenylhydrazone. Cancer Res. 1989 Aug 15;49(16):4477–4482. [PubMed] [Google Scholar]
- Newell K., Wood P., Stratford I., Tannock I. Effects of agents which inhibit the regulation of intracellular pH on murine solid tumours. Br J Cancer. 1992 Aug;66(2):311–317. doi: 10.1038/bjc.1992.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Wan P., Grinstein S., Tannock I. Cytotoxicity of compounds that interfere with the regulation of intracellular pH: a potential new class of anticancer drugs. Cancer Res. 1987 Mar 15;47(6):1497–1504. [PubMed] [Google Scholar]
- Rotstein O. D., Nasmith P. E., Grinstein S. The Bacteroides by-product succinic acid inhibits neutrophil respiratory burst by reducing intracellular pH. Infect Immun. 1987 Apr;55(4):864–870. doi: 10.1128/iai.55.4.864-870.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T. L. The transport and oxidation of succinate by Ehrlich ascites-tumour cells. Biochem J. 1976 Oct 15;160(1):121–123. doi: 10.1042/bj1600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen R. G. Response of solid tumors to chemotherapy monitored by in vivo 31P nuclear magnetic resonance spectroscopy: a review. Cancer Res. 1989 Aug 1;49(15):4075–4085. [PubMed] [Google Scholar]
- Tannock I. F., Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989 Aug 15;49(16):4373–4384. [PubMed] [Google Scholar]
- Thomas C., Counsell C., Wood P., Adams G. E. Use of fluorine-19 nuclear magnetic resonance spectroscopy and hydralazine for measuring dynamic changes in blood perfusion volume in tumors in mice. J Natl Cancer Inst. 1992 Feb 5;84(3):174–180. doi: 10.1093/jnci/84.3.174. [DOI] [PubMed] [Google Scholar]
- Thomson J. E., Rauth A. M. An in vitro assay to measure the viability of KHT tumor cells not previously exposed to culture conditions. Radiat Res. 1974 May;58(2):262–276. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984 Dec;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]



