Abstract
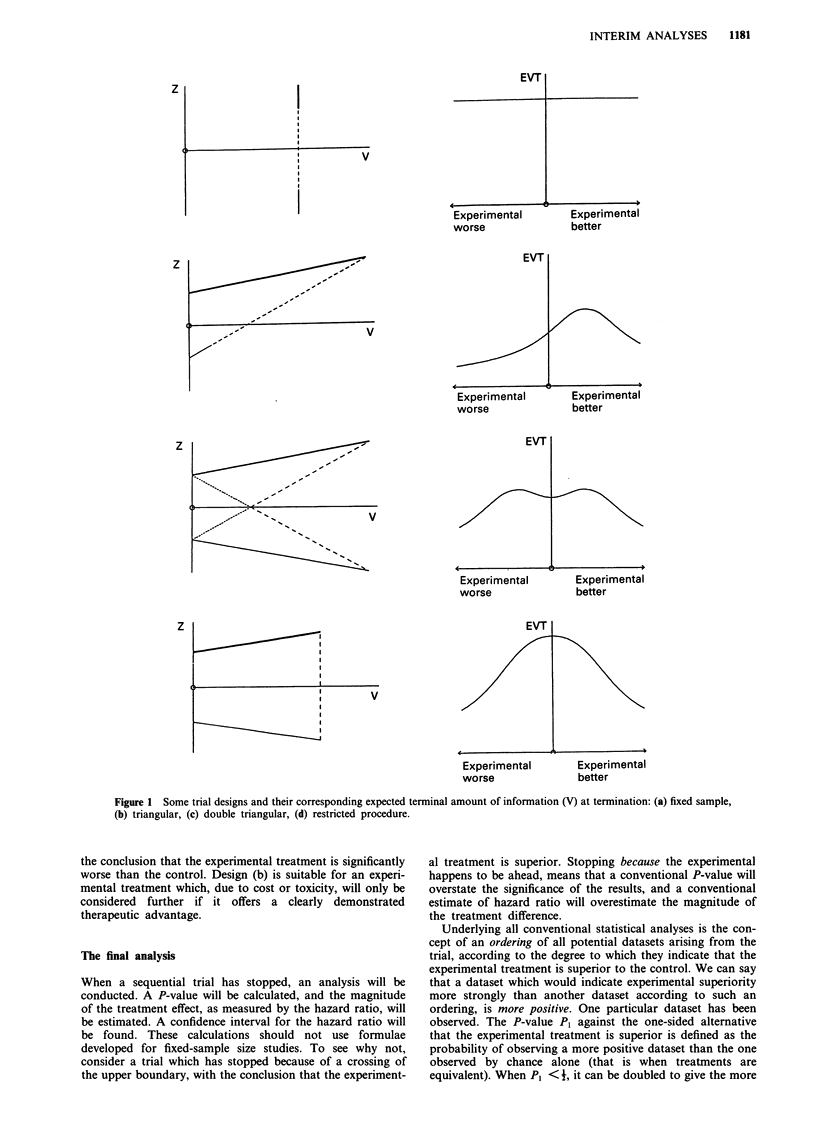
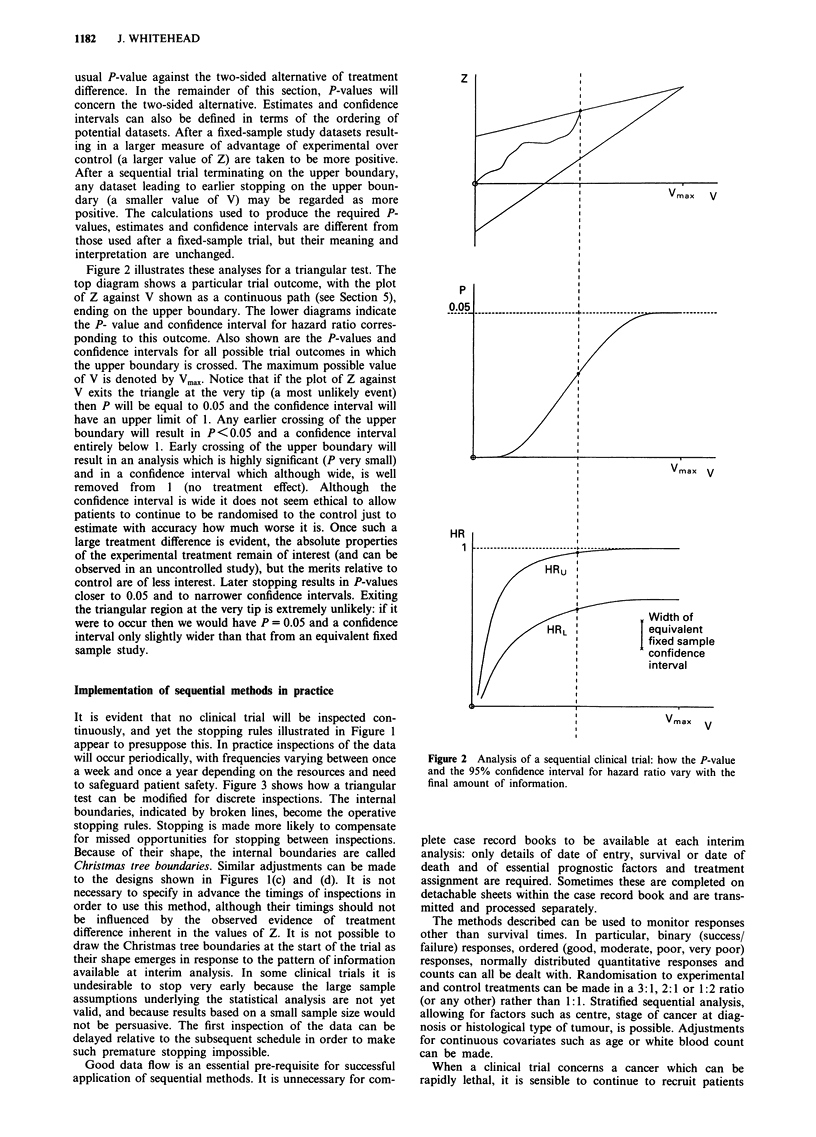
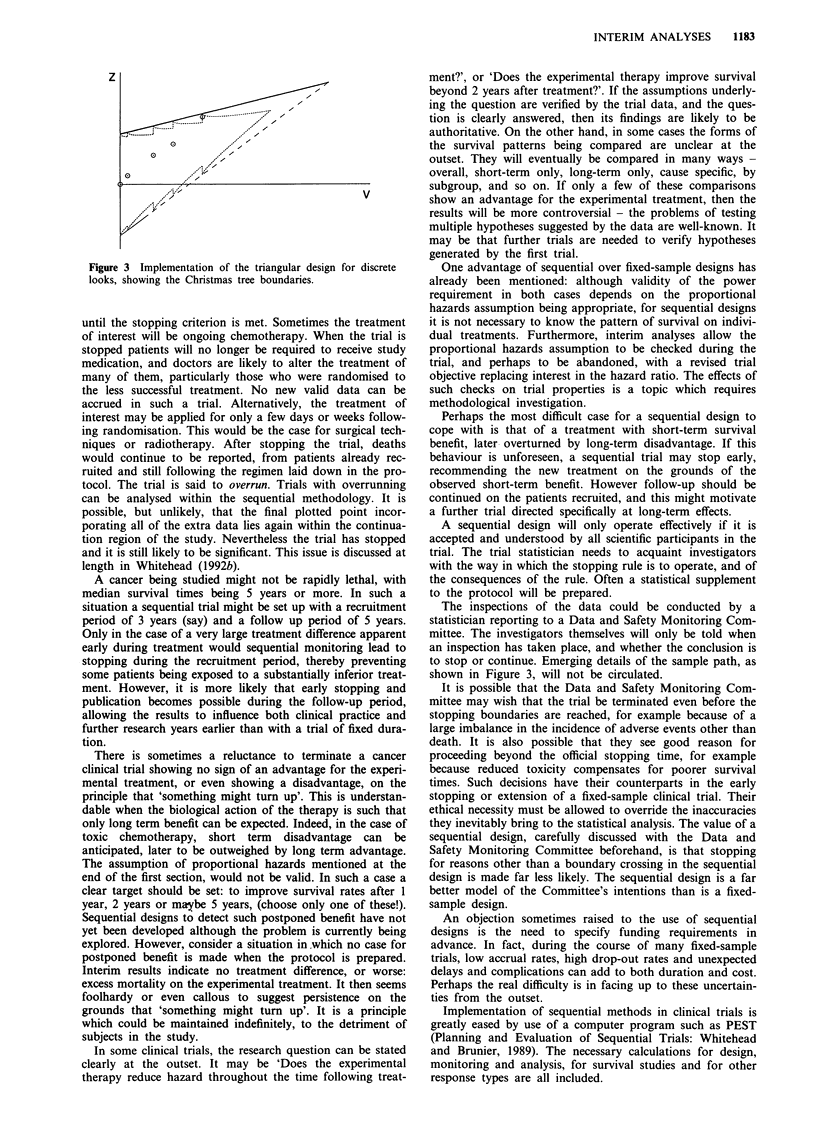
A clinical trial conducted according to a schedule of interim analyses written into the protocol, and stopped according to a predetermined rule, is known to statisticians as a sequential clinical trial. This methodology is becoming more widely used in trials concerning life-threatening diseases because of its ability to adjust the sample size to the emerging information on treatment efficacy. When treatments under comparison differ appreciably, small samples will be sufficient; for more subtle differences larger numbers of patients need to be recruited. Sequential methods have already been used in certain cancer clinical trials, and they are especially appropriate for such studies. In this paper the principles of sample size determination are reviewed, and the essential aspects of designing sequential trials are described. The necessity for a special form of statistical analysis following a sequential trial is explained, and the consequences of early or late stopping on the analysis are investigated. Compromises which have to be made between the formal requirements of theory and the practical realities of trial conduct are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry D. A. Monitoring accumulating data in a clinical trial. Biometrics. 1989 Dec;45(4):1197–1211. [PubMed] [Google Scholar]
- Facey K. M., Whitehead J. The impact that group sequential tests would have made on ECOG clinical trials. Stat Med. 1990 Jul;9(7):853–854. doi: 10.1002/sim.4780090716. [DOI] [PubMed] [Google Scholar]
- Freedman L. S., Spiegelhalter D. J. Comparison of Bayesian with group sequential methods for monitoring clinical trials. Control Clin Trials. 1989 Dec;10(4):357–367. doi: 10.1016/0197-2456(89)90001-9. [DOI] [PubMed] [Google Scholar]
- Geller N. L., Pocock S. J. Interim analyses in randomized clinical trials: ramifications and guidelines for practitioners. Biometrics. 1987 Mar;43(1):213–223. [PubMed] [Google Scholar]
- Jones D. R., Newman C. E., Whitehead J. The design of a sequential clinical trial for the comparison of two lung cancer treatments. Stat Med. 1982 Jan-Mar;1(1):73–82. doi: 10.1002/sim.4780010110. [DOI] [PubMed] [Google Scholar]
- KILPATRICK G. S., OLDHAM P. D. Calcium chloride and adrenaline as bronchial dilators compared by sequential analysis. Br Med J. 1954 Dec 11;2(4901):1388–1391. doi: 10.1136/bmj.2.4901.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J. S., Lawson L. M., Levitt N., Belzberg A., Schechter M. T., Ruedy J. Corticosteroids prevent early deterioration in patients with moderately severe Pneumocystis carinii pneumonia and the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1990 Jul 1;113(1):14–20. doi: 10.7326/0003-4819-113-1-14. [DOI] [PubMed] [Google Scholar]
- Newman C. E., Cox R., Ford C. H., Johnson J. R., Jones D. R., Wheaton M. Reduced survival with radiotherapy and razoxane compared with radiotherapy alone for inoperable lung cancer in a randomised double-blind trial. Br J Cancer. 1985 May;51(5):731–732. doi: 10.1038/bjc.1985.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien P. C., Fleming T. R. A multiple testing procedure for clinical trials. Biometrics. 1979 Sep;35(3):549–556. [PubMed] [Google Scholar]
- Pocock S. J. Interim analyses for randomized clinical trials: the group sequential approach. Biometrics. 1982 Mar;38(1):153–162. [PubMed] [Google Scholar]
- Pocock S. J. When to stop a clinical trial. BMJ. 1992 Jul 25;305(6847):235–240. doi: 10.1136/bmj.305.6847.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner G. L., Tsiatis A. A. The impact that group sequential tests would have made on ECOG clinical trials. Stat Med. 1989 Apr;8(4):505–516. doi: 10.1002/sim.4780080413. [DOI] [PubMed] [Google Scholar]
- Storb R., Deeg H. J., Whitehead J., Appelbaum F., Beatty P., Bensinger W., Buckner C. D., Clift R., Doney K., Farewell V. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986 Mar 20;314(12):729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- Whitehead J., Jones D. R., Ellis S. H. The analysis of a sequential clinical trial for the comparison of two lung cancer treatments. Stat Med. 1983 Apr-Jun;2(2):183–190. doi: 10.1002/sim.4780020212. [DOI] [PubMed] [Google Scholar]
- Whitehead J. Overrunning and underrunning in sequential clinical trials. Control Clin Trials. 1992 Apr;13(2):106–121. doi: 10.1016/0197-2456(92)90017-t. [DOI] [PubMed] [Google Scholar]



