Abstract
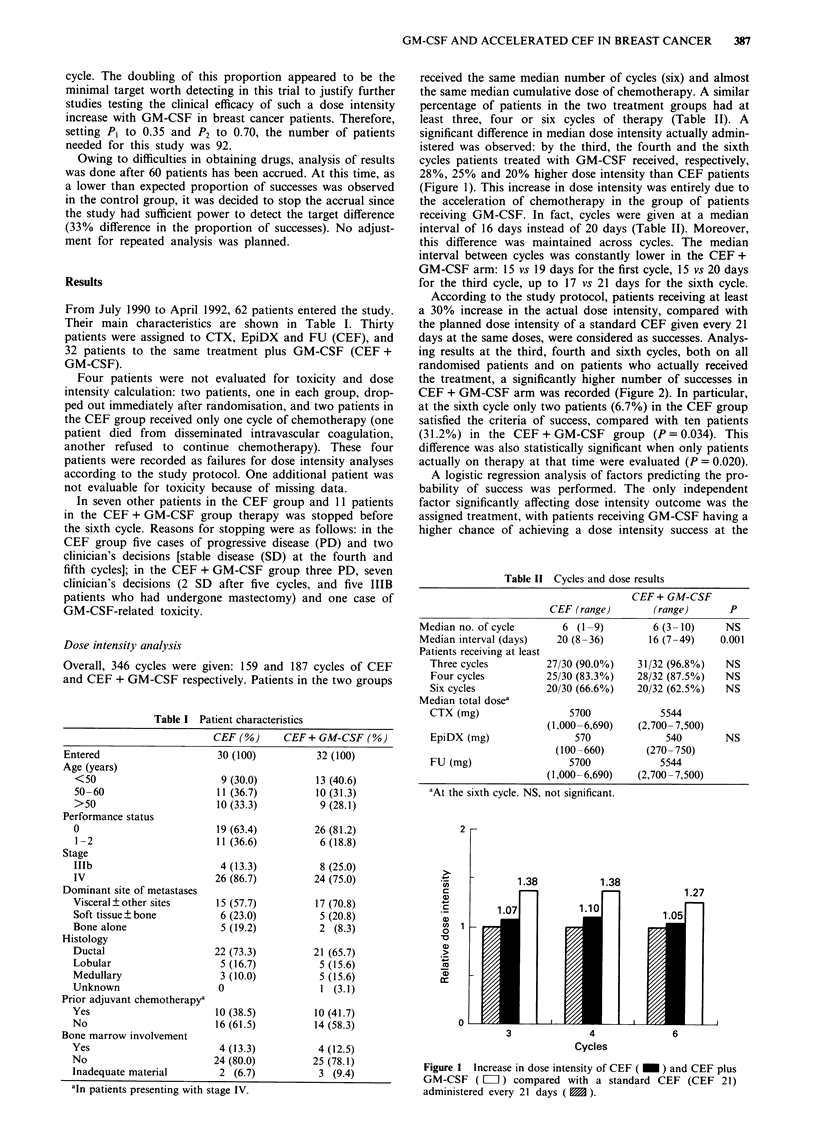
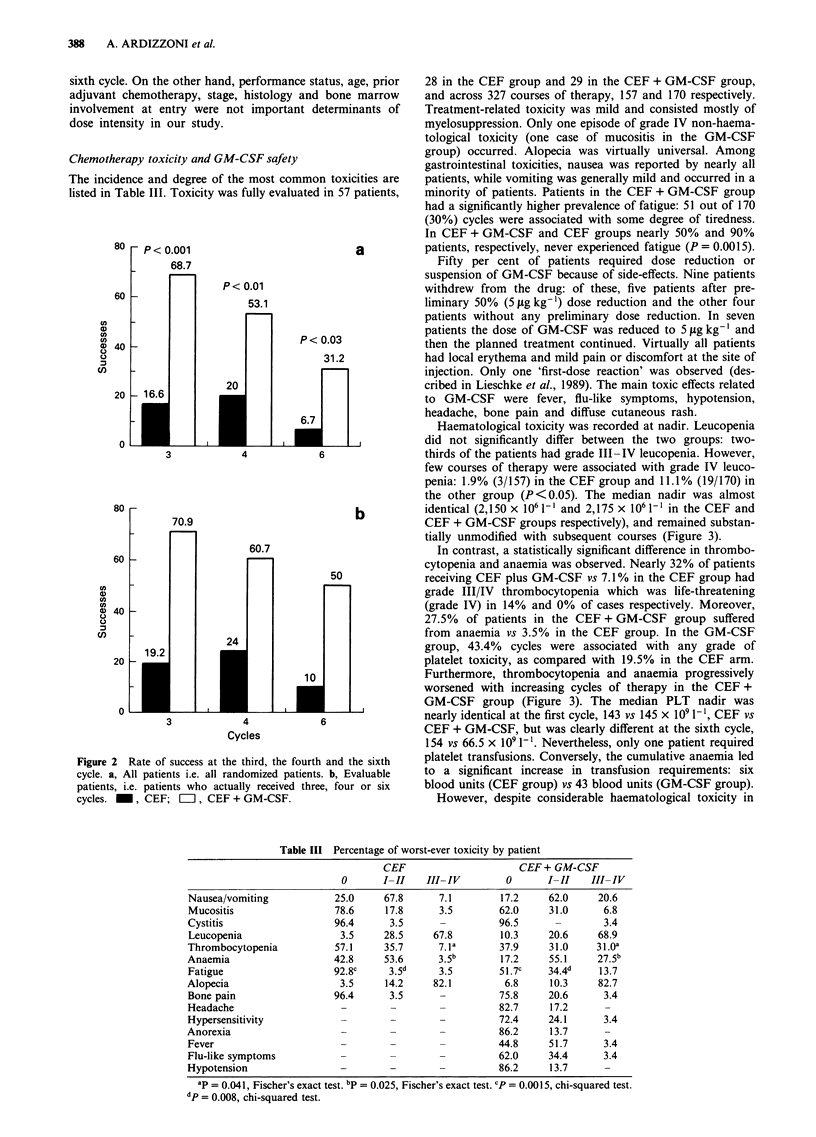
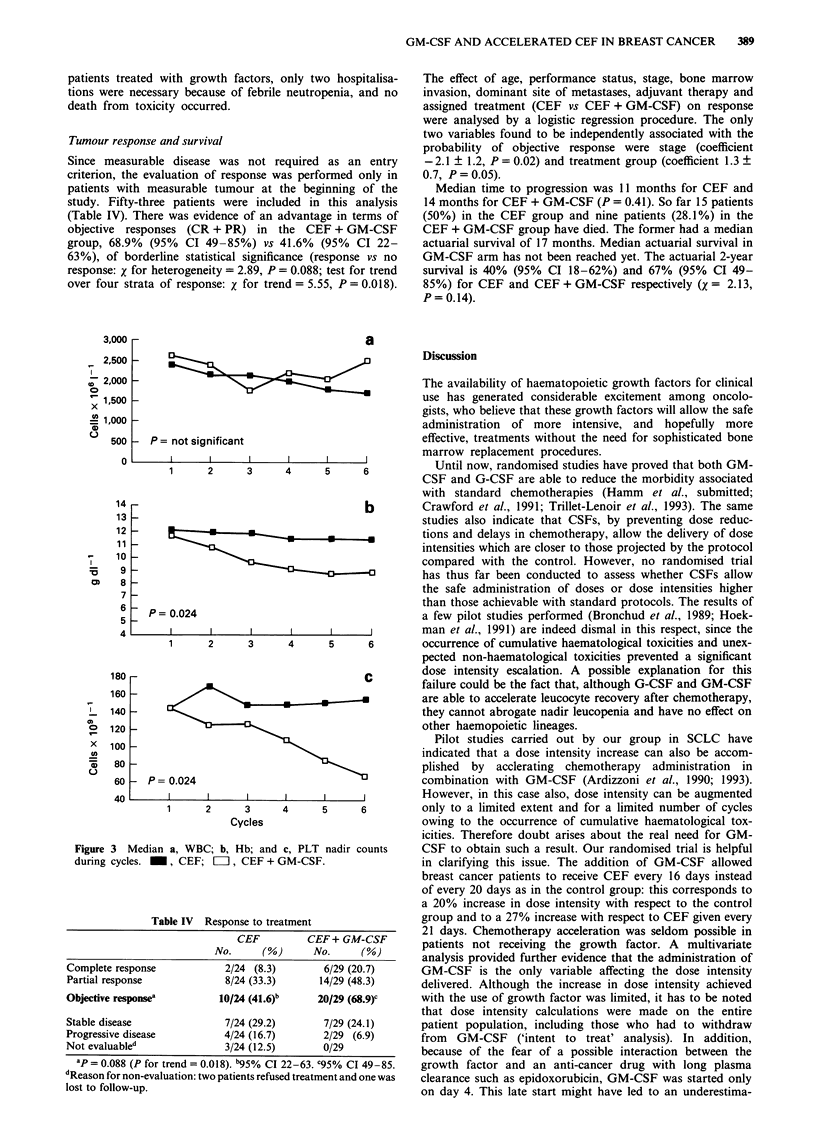
A randomised study was conducted in 62 patients with advanced breast cancer to assess whether granulocyte-macrophage colony-stimulating factor (GM-CSF) would yield an increase in the dose intensity of a standard-dose CEF regimen through an acceleration of chemotherapy administration. Patients received CEF (cyclophosphamide 600 mg m-2, epidoxorubicin 60 mg m-2 and fluorouracil 600 mg m-2) i.v. on day 1 or the same chemotherapy, plus GM-CSF 10 micrograms kg-1 s.c. starting from day 4, repeated as soon as haematopoietic recovery from nadir occurred. Patients in the CEF + GM-CSF group received chemotherapy at a median interval of 16 days compared with 20 days in the control group. This led to a significant increase (P = 0.02) in the dose intensity actually administered in the third, fourth and sixth cycles: +28%, +25%, +20% respectively. Non-haematological toxicity was mild. GM-CSF had to be reduced or suspended in 50% of patients because of toxicity. Haematological toxicity, mainly cumulative anaemia and thrombocytopenia, was manageable. An increase in response rate for patients with measurable disease, of borderline statistical significance (P = 0.088, P for trend = 0.018), from 42% in the CEF group to 69% in the CEF + GM-CSF group, was observed. This randomised trial indicates that GM-CSF is useful for chemotherapy acceleration. Accelerated CEF + GM-CSF is a moderately dose-intensive regimen that can be administered in an outpatient clinic and is associated with a high objective response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardizzoni A., Sertoli M. R., Corcione A., Pennucci M. C., Baldini E., Intra E., Ferrarini M., Rosso R., Mazzanti P., Pistoia V. Accelerated chemotherapy with or without GM-CSF for small cell lung cancer: a non-randomised pilot study. Eur J Cancer. 1990;26(9):937–941. doi: 10.1016/0277-5379(90)90614-y. [DOI] [PubMed] [Google Scholar]
- Ardizzoni A., Venturini M., Crinò L., Sertoli M. R., Bruzzi P., Pennucci M. C., Mariani G. L., Garrone O., Bracarda S., Rosso R. High dose-intensity chemotherapy, with accelerated cyclophosphamide-doxorubicin-etoposide and granulocyte-macrophage colony stimulating factor, in the treatment of small cell lung cancer. Eur J Cancer. 1993;29A(5):687–692. doi: 10.1016/s0959-8049(05)80347-8. [DOI] [PubMed] [Google Scholar]
- Bonadonna G., Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med. 1981 Jan 1;304(1):10–15. doi: 10.1056/NEJM198101013040103. [DOI] [PubMed] [Google Scholar]
- Bronchud M. H., Howell A., Crowther D., Hopwood P., Souza L., Dexter T. M. The use of granulocyte colony-stimulating factor to increase the intensity of treatment with doxorubicin in patients with advanced breast and ovarian cancer. Br J Cancer. 1989 Jul;60(1):121–125. doi: 10.1038/bjc.1989.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Pereira J., Costa F. O., Henriques E., Godinho F., Cantinho-Lopes M. G., Sales-Luis A., Rubens R. D. A comparison of two doses of adriamycin in the primary chemotherapy of disseminated breast carcinoma. Br J Cancer. 1987 Oct;56(4):471–473. doi: 10.1038/bjc.1987.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte P. F., Pronzato P., Rubagotti A., Alama A., Amadori D., Demicheli R., Gardin G., Gentilini P., Jacomuzzi A., Lionetto R. Conventional versus cytokinetic polychemotherapy with estrogenic recruitment in metastatic breast cancer: results of a randomized cooperative trial. J Clin Oncol. 1987 Mar;5(3):339–347. doi: 10.1200/JCO.1987.5.3.339. [DOI] [PubMed] [Google Scholar]
- Crawford J., Ozer H., Stoller R., Johnson D., Lyman G., Tabbara I., Kris M., Grous J., Picozzi V., Rausch G. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991 Jul 18;325(3):164–170. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- Demetri G. D., Antman K. H. Granulocyte-macrophage colony-stimulating factor (GM-CSF): preclinical and clinical investigations. Semin Oncol. 1992 Aug;19(4):362–385. [PubMed] [Google Scholar]
- Ebbs S. R., Saunders J. A., Graham H., A'Hern R. P., Bates T., Baum M. Advanced breast cancer. A randomised trial of epidoxorubicin at two different dosages and two administration systems. Acta Oncol. 1989;28(6):887–892. doi: 10.3109/02841868909092326. [DOI] [PubMed] [Google Scholar]
- Forastiere A. A., Hakes T. B., Wittes J. T., Wittes R. E. Cisplatin in the treatment of metastatic breast carcinoma: A prospective randomized trial of two dosage schedules. Am J Clin Oncol. 1982 Jun;5(3):243–247. doi: 10.1097/00000421-198206000-00001. [DOI] [PubMed] [Google Scholar]
- Gabrilove J. L., Jakubowski A., Scher H., Sternberg C., Wong G., Grous J., Yagoda A., Fain K., Moore M. A., Clarkson B. Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N Engl J Med. 1988 Jun 2;318(22):1414–1422. doi: 10.1056/NEJM198806023182202. [DOI] [PubMed] [Google Scholar]
- Habeshaw T., Paul J., Jones R., Stallard S., Stewart M., Kaye S. B., Soukop M., Symonds R. P., Reed N. S., Rankin E. M. Epirubicin at two dose levels with prednisolone as treatment for advanced breast cancer: the results of a randomized trial. J Clin Oncol. 1991 Feb;9(2):295–304. doi: 10.1200/JCO.1991.9.2.295. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Hayes D. F., Gelman R. Dose-response in the treatment of breast cancer: a critical review. J Clin Oncol. 1988 Sep;6(9):1501–1515. doi: 10.1200/JCO.1988.6.9.1501. [DOI] [PubMed] [Google Scholar]
- Hoekman K., Wagstaff J., van Groeningen C. J., Vermorken J. B., Boven E., Pinedo H. M. Effects of recombinant human granulocyte-macrophage colony-stimulating factor on myelosuppression induced by multiple cycles of high-dose chemotherapy in patients with advanced breast cancer. J Natl Cancer Inst. 1991 Nov 6;83(21):1546–1553. doi: 10.1093/jnci/83.21.1546. [DOI] [PubMed] [Google Scholar]
- Hoogstraten B., George S. L., Samal B., Rivkin S. E., Costanzi J. J., Bonnet J. D., Thigpen T., Braine H. Combination chemotherapy and adriamycin in patients with advanced breast cancer. A Southwest Oncology Group study. Cancer. 1976 Jul;38(1):13–20. doi: 10.1002/1097-0142(197607)38:1<13::aid-cncr2820380104>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Hortobagyi G. N., Bodey G. P., Buzdar A. U., Frye D., Legha S. S., Malik R., Smith T. L., Blumenschein G. R., Yap H. Y., Rodriguez V. Evaluation of high-dose versus standard FAC chemotherapy for advanced breast cancer in protected environment units: a prospective randomized study. J Clin Oncol. 1987 Mar;5(3):354–364. doi: 10.1200/JCO.1987.5.3.354. [DOI] [PubMed] [Google Scholar]
- Hryniuk W., Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984 Nov;2(11):1281–1288. doi: 10.1200/JCO.1984.2.11.1281. [DOI] [PubMed] [Google Scholar]
- Kaplan L. D., Kahn J. O., Crowe S., Northfelt D., Neville P., Grossberg H., Abrams D. I., Tracey J., Mills J., Volberding P. A. Clinical and virologic effects of recombinant human granulocyte-macrophage colony-stimulating factor in patients receiving chemotherapy for human immunodeficiency virus-associated non-Hodgkin's lymphoma: results of a randomized trial. J Clin Oncol. 1991 Jun;9(6):929–940. doi: 10.1200/JCO.1991.9.6.929. [DOI] [PubMed] [Google Scholar]
- Lieschke G. J., Cebon J., Morstyn G. Characterization of the clinical effects after the first dose of bacterially synthesized recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1989 Dec;74(8):2634–2643. [PubMed] [Google Scholar]
- Logothetis C. J., Dexeus F. H., Sella A., Amato R. J., Kilbourn R. G., Finn L., Gutterman J. U. Escalated therapy for refractory urothelial tumors: methotrexate-vinblastine-doxorubicin-cisplatin plus unglycosylated recombinant human granulocyte-macrophage colony-stimulating factor. J Natl Cancer Inst. 1990 Apr 18;82(8):667–672. doi: 10.1093/jnci/82.8.667. [DOI] [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- O'Bryan R. M., Baker L. H., Gottlieb J. E., Rivkin S. E., Balcerzak S. P., Grumet G. N., Salmon S. E., Moon T. E., Hoogstraten B. Dose response evaluation of adriamycin in human neoplasia. Cancer. 1977 May;39(5):1940–1948. doi: 10.1002/1097-0142(197705)39:5<1940::aid-cncr2820390505>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Skipper H. E. Dose intensity versus total dose of chemotherapy: an experimental basis. Important Adv Oncol. 1990:43–64. [PubMed] [Google Scholar]
- Tannock I. F., Boyd N. F., DeBoer G., Erlichman C., Fine S., Larocque G., Mayers C., Perrault D., Sutherland H. A randomized trial of two dose levels of cyclophosphamide, methotrexate, and fluorouracil chemotherapy for patients with metastatic breast cancer. J Clin Oncol. 1988 Sep;6(9):1377–1387. doi: 10.1200/JCO.1988.6.9.1377. [DOI] [PubMed] [Google Scholar]
- Trillet-Lenoir V., Green J., Manegold C., Von Pawel J., Gatzemeier U., Lebeau B., Depierre A., Johnson P., Decoster G., Tomita D. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer. 1993;29A(3):319–324. doi: 10.1016/0959-8049(93)90376-q. [DOI] [PubMed] [Google Scholar]



