Abstract
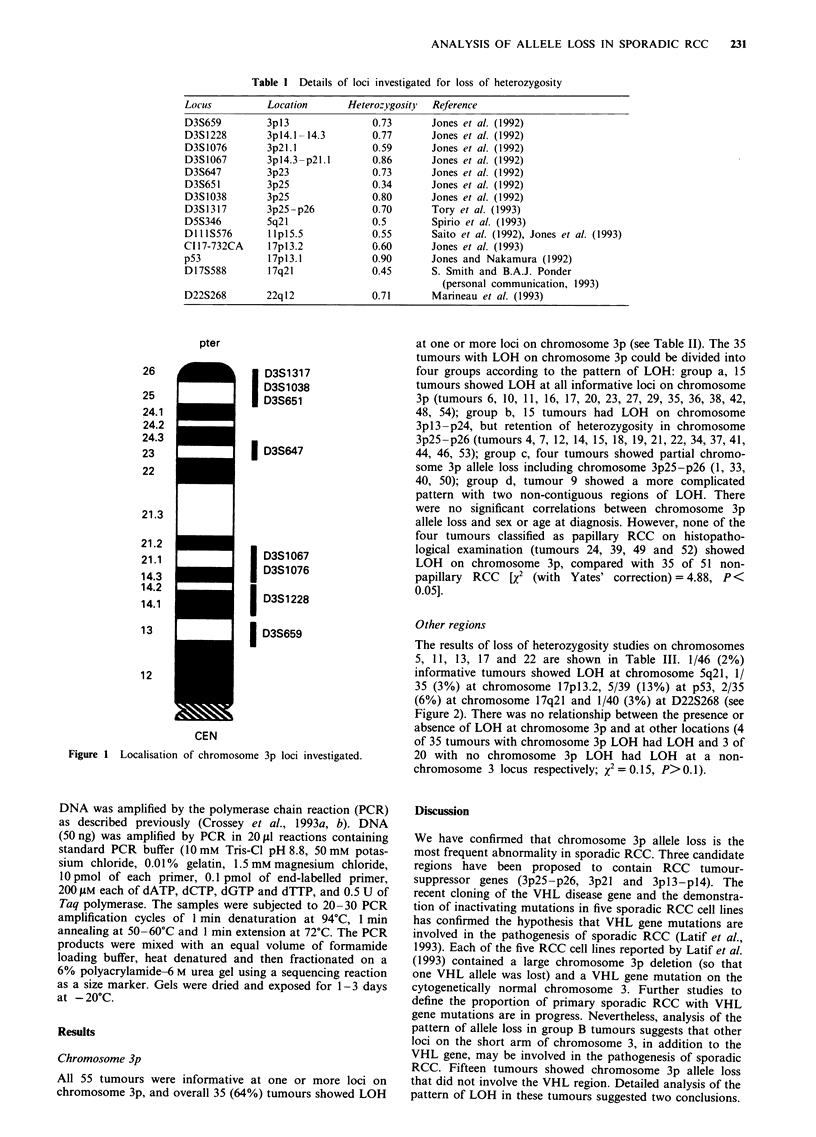
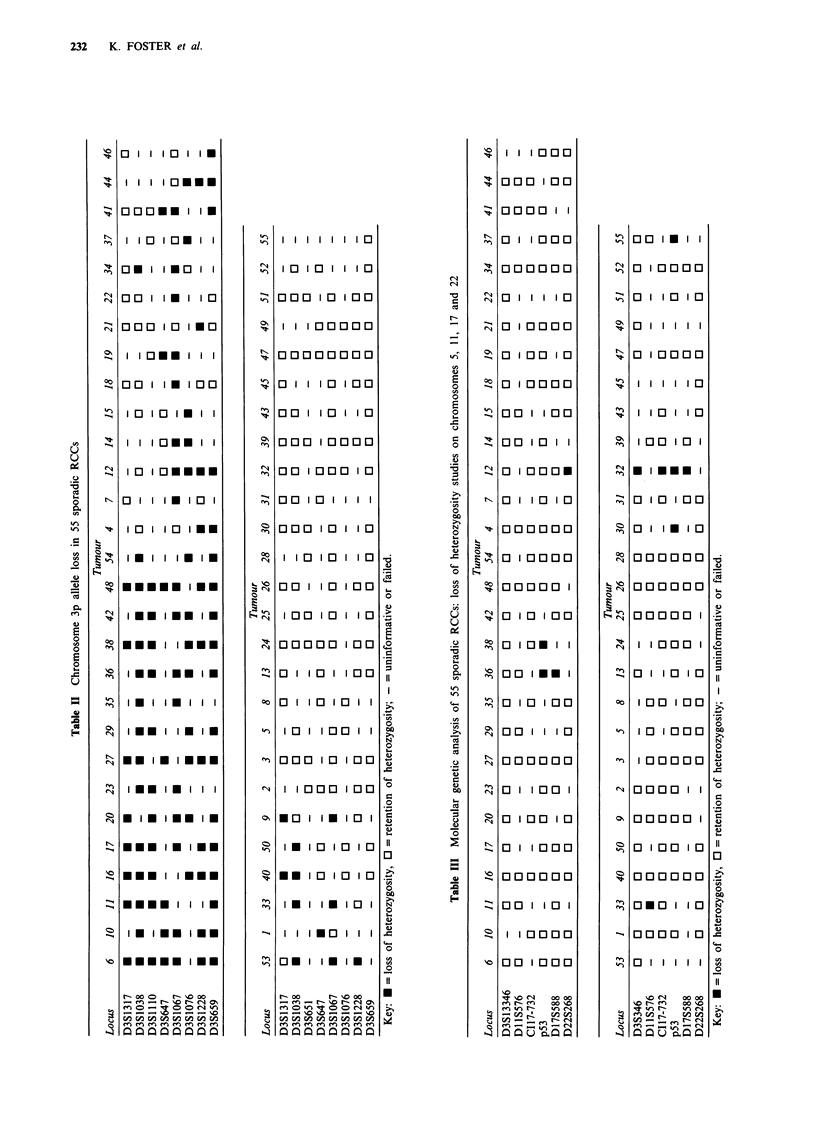
To investigate the role of tumour-suppressor genes on the short arm of chromosome 3 in the mechanism of tumorigenesis in non-familial renal cell carcinoma, we analysed 55 paired blood-tumour DNA samples for allele loss on chromosome 3p and in the region of known or putative tumour-suppressor genes on chromosomes 5, 11, 17 and 22. Sixty-four per cent (35/55) of informative tumours showed loss of heterozygosity (LOH) of at least one locus on the short arm of chromosome 3, compared with only 13% at the p53 tumour-suppressor gene and 6% at 17q21. LOH at chromosome 5q21 and 22q was uncommon (2-3%). Detailed analysis of the regions of LOH on chromosome 3p suggested that, in addition to the VHL gene in chromosome 3p25-p26, mutations in one or more tumour-suppressor genes in chromosome 3p13-p24 may be involved in the pathogenesis of sporadic renal cell carcinoma (RCC). We also confirmed previous suggestions that chromosome 3p allele loss is not a feature of papillary RCC (P < 0.05).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglard P., Tory K., Brauch H., Weiss G. H., Latif F., Merino M. J., Lerman M. I., Zbar B., Linehan W. M. Molecular analysis of genetic changes in the origin and development of renal cell carcinoma. Cancer Res. 1991 Feb 15;51(4):1071–1077. [PubMed] [Google Scholar]
- Bergerheim U., Nordenskjöld M., Collins V. P. Deletion mapping in human renal cell carcinoma. Cancer Res. 1989 Mar 15;49(6):1390–1396. [PubMed] [Google Scholar]
- Cohen A. J., Li F. P., Berg S., Marchetto D. J., Tsai S., Jacobs S. C., Brown R. S. Hereditary renal-cell carcinoma associated with a chromosomal translocation. N Engl J Med. 1979 Sep 13;301(11):592–595. doi: 10.1056/NEJM197909133011107. [DOI] [PubMed] [Google Scholar]
- Crossey P. A., Maher E. R., Jones M. H., Richards F. M., Latif F., Phipps M. E., Lush M., Foster K., Tory K., Green J. S. Genetic linkage between von Hippel-Lindau disease and three microsatellite polymorphisms refines the localisation of the VHL locus. Hum Mol Genet. 1993 Mar;2(3):279–282. doi: 10.1093/hmg/2.3.279. [DOI] [PubMed] [Google Scholar]
- Horii A., Nakatsuru S., Miyoshi Y., Ichii S., Nagase H., Ando H., Yanagisawa A., Tsuchiya E., Kato Y., Nakamura Y. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res. 1992 Dec 1;52(23):6696–6698. [PubMed] [Google Scholar]
- Hosoe S., Brauch H., Latif F., Glenn G., Daniel L., Bale S., Choyke P., Gorin M., Oldfield E., Berman A. Localization of the von Hippel-Lindau disease gene to a small region of chromosome 3. Genomics. 1990 Dec;8(4):634–640. doi: 10.1016/0888-7543(90)90249-t. [DOI] [PubMed] [Google Scholar]
- Jones M. H., Nakamura Y. Detection of loss of heterozygosity at the human TP53 locus using a dinucleotide repeat polymorphism. Genes Chromosomes Cancer. 1992 Jul;5(1):89–90. doi: 10.1002/gcc.2870050113. [DOI] [PubMed] [Google Scholar]
- Jones M. H., Yamakawa K., Nakamura Y. Isolation and characterization of 19 dinucleotide repeat polymorphisms on chromosome 3p. Hum Mol Genet. 1992 May;1(2):131–133. doi: 10.1093/hmg/1.2.131. [DOI] [PubMed] [Google Scholar]
- Kovacs G., Erlandsson R., Boldog F., Ingvarsson S., Müller-Brechlin R., Klein G., Sümegi J. Consistent chromosome 3p deletion and loss of heterozygosity in renal cell carcinoma. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1571–1575. doi: 10.1073/pnas.85.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G., Wilkens L., Papp T., de Riese W. Differentiation between papillary and nonpapillary renal cell carcinomas by DNA analysis. J Natl Cancer Inst. 1989 Apr 5;81(7):527–530. doi: 10.1093/jnci/81.7.527. [DOI] [PubMed] [Google Scholar]
- Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Maher E. R., Bentley E., Yates J. R., Latif F., Lerman M., Zbar B., Affara N. A., Ferguson-Smith M. A. Mapping of the von Hippel-Lindau disease locus to a small region of chromosome 3p by genetic linkage analysis. Genomics. 1991 Aug;10(4):957–960. doi: 10.1016/0888-7543(91)90185-h. [DOI] [PubMed] [Google Scholar]
- Maher E. R., Yates J. R. Familial renal cell carcinoma: clinical and molecular genetic aspects. Br J Cancer. 1991 Feb;63(2):176–179. doi: 10.1038/bjc.1991.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher E. R., Yates J. R., Ferguson-Smith M. A. Statistical analysis of the two stage mutation model in von Hippel-Lindau disease, and in sporadic cerebellar haemangioblastoma and renal cell carcinoma. J Med Genet. 1990 May;27(5):311–314. doi: 10.1136/jmg.27.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher E. R., Yates J. R., Harries R., Benjamin C., Harris R., Moore A. T., Ferguson-Smith M. A. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990 Nov;77(283):1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- Marineau C., Baron C., Delattre O., Zucman J., Thomas G., Rouleau G. A. Dinucleotide repeat polymorphism at the D22S268 locus. Hum Mol Genet. 1993 Mar;2(3):336–336. doi: 10.1093/hmg/2.3.336-a. [DOI] [PubMed] [Google Scholar]
- Morita R., Ishikawa J., Tsutsumi M., Hikiji K., Tsukada Y., Kamidono S., Maeda S., Nakamura Y. Allelotype of renal cell carcinoma. Cancer Res. 1991 Feb 1;51(3):820–823. [PubMed] [Google Scholar]
- Richards F. M., Maher E. R., Latif F., Phipps M. E., Tory K., Lush M., Crossey P. A., Oostra B., Enblad P., Gustavson K. H. Detailed genetic mapping of the von Hippel-Lindau disease tumour suppressor gene. J Med Genet. 1993 Feb;30(2):104–107. doi: 10.1136/jmg.30.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S., Okui K., Tokino T., Oshimura M., Nakamura Y. Isolation and mapping of 68 RFLP markers on human chromosome 6. Am J Hum Genet. 1992 Jan;50(1):65–70. [PMC free article] [PubMed] [Google Scholar]
- Seizinger B. R., Rouleau G. A., Ozelius L. J., Lane A. H., Farmer G. E., Lamiell J. M., Haines J., Yuen J. W., Collins D., Majoor-Krakauer D. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988 Mar 17;332(6161):268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Smith D. I., Filling-Katz M. R., Neumann H., Green J. S., Choyke P. L., Anderson K. M., Freiman R. N., Klauck S. M., Whaley J. Genetic flanking markers refine diagnostic criteria and provide insights into the genetics of Von Hippel Lindau disease. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2864–2868. doi: 10.1073/pnas.88.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Yokota J., Mori N., Shuin T., Shinoda M., Terada M., Oshimura M. Introduction of normal chromosome 3p modulates the tumorigenicity of a human renal cell carcinoma cell line YCR. Oncogene. 1990 Feb;5(2):185–194. [PubMed] [Google Scholar]
- Spirio L., Nelson L., Ward K., Burt R., White R., Leppert M. A CA-repeat polymorphism close to the adenomatous polyposis coli (APC) gene offers improved diagnostic testing for familial APC. Am J Hum Genet. 1993 Feb;52(2):286–296. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Tamura G., Satodate R., Fujioka T. Infrequent mutation of p53 gene in human renal cell carcinoma detected by polymerase chain reaction single-strand conformation polymorphism analysis. Jpn J Cancer Res. 1992 Mar;83(3):233–235. doi: 10.1111/j.1349-7006.1992.tb00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe S., Shuin T., Kubota Y., Horikoshi T., Danenberg K., Danenberg P. V. p53 gene mutation in primary human renal cell carcinoma. Oncol Res. 1992;4(11-12):467–472. [PubMed] [Google Scholar]
- Tory K., Brauch H., Linehan M., Barba D., Oldfield E., Filling-Katz M., Seizinger B., Nakamura Y., White R., Marshall F. F. Specific genetic change in tumors associated with von Hippel-Lindau disease. J Natl Cancer Inst. 1989 Jul 19;81(14):1097–1101. doi: 10.1093/jnci/81.14.1097. [DOI] [PubMed] [Google Scholar]
- Tory K., Latif F., Modi W., Schmidt L., Wei M. H., Li H., Cobler P., Orcutt M. L., Delisio J., Geil L. A genetic linkage map of 96 loci on the short arm of human chromosome 3. Genomics. 1992 Jun;13(2):275–286. doi: 10.1016/0888-7543(92)90243-l. [DOI] [PubMed] [Google Scholar]
- Yamakawa K., Morita R., Takahashi E., Hori T., Ishikawa J., Nakamura Y. A detailed deletion mapping of the short arm of chromosome 3 in sporadic renal cell carcinoma. Cancer Res. 1991 Sep 1;51(17):4707–4711. [PubMed] [Google Scholar]
- Yamakawa K., Takahashi E., Murata M., Okui K., Yokoyama S., Nakamura Y. Detailed mapping around the breakpoint of (3;8) translocation in familial renal cell carcinoma and FRA3B. Genomics. 1992 Oct;14(2):412–416. doi: 10.1016/s0888-7543(05)80234-4. [DOI] [PubMed] [Google Scholar]
- Zbar B., Brauch H., Talmadge C., Linehan M. Loss of alleles of loci on the short arm of chromosome 3 in renal cell carcinoma. 1987 Jun 25-Jul 1Nature. 327(6124):721–724. doi: 10.1038/327721a0. [DOI] [PubMed] [Google Scholar]
- van der Hout A. H., van der Vlies P., Wijmenga C., Li F. P., Oosterhuis J. W., Buys C. H. The region of common allelic losses in sporadic renal cell carcinoma is bordered by the loci D3S2 and THRB. Genomics. 1991 Nov;11(3):537–542. doi: 10.1016/0888-7543(91)90060-r. [DOI] [PubMed] [Google Scholar]




