Abstract
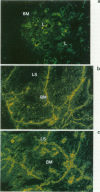
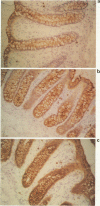
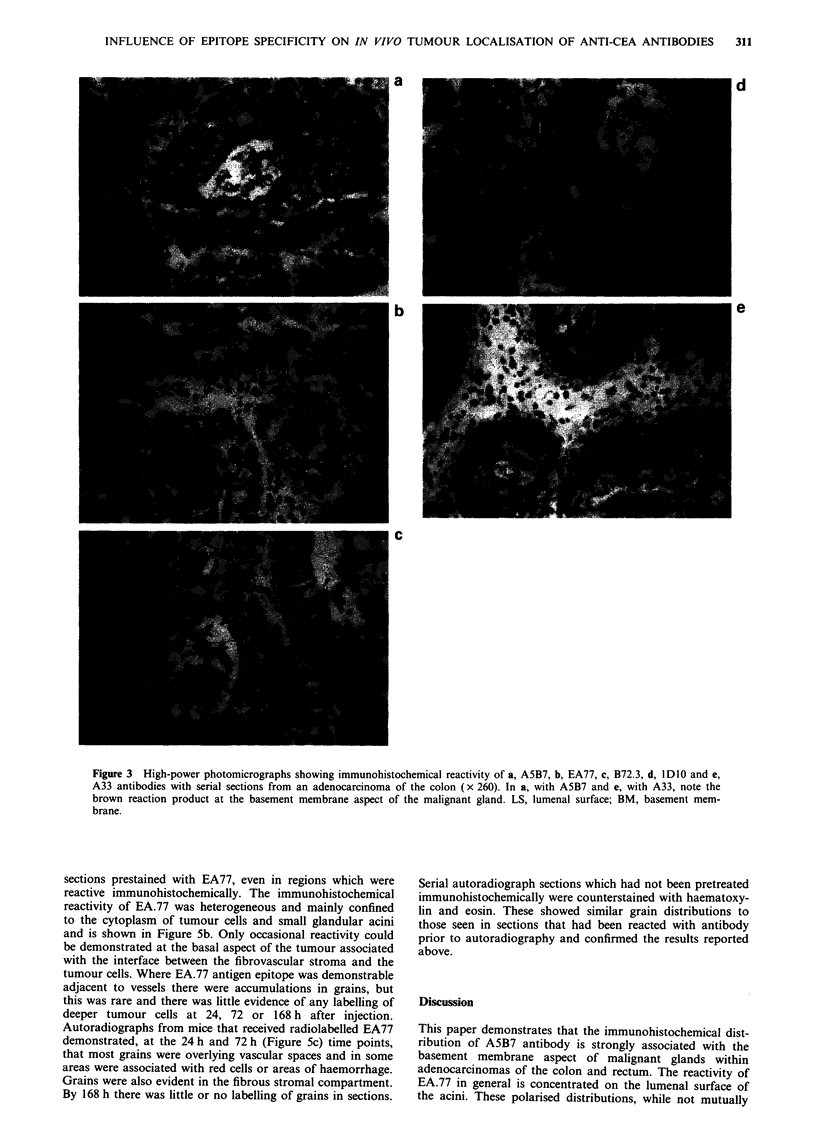
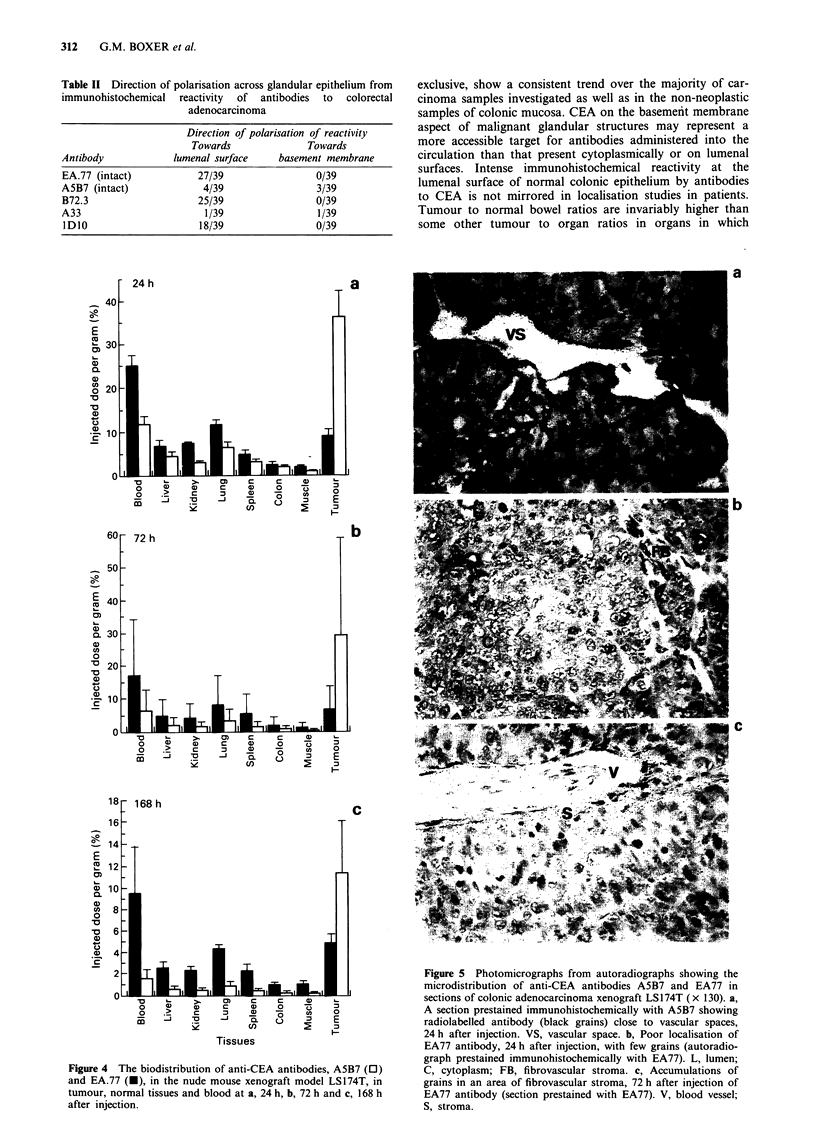
Antibody targeting has potential for selective delivery of cancer therapy. However, there is a wide variation in the degree of antibody localisation in individual patients with colorectal adenocarcinoma. Colorectal adenocarcinomas are composed of glandular structures separated from fibrovascular stroma by a basal lamina which may represent a significant barrier to extravasated antibody. Basement membrane-associated CEA epitopes may be more accessible to antibodies than those which are cytoplasmic or lumenal. We have investigated by immunohistochemistry and in vivo localisation, the extent to which distribution of antigen epitopes influences targeting. Two monoclonal antibodies (A5B7 and EA77) recognising non-overlapping CEA epitopes were reacted immunohistochemically with samples of 39 tumours. Intensity and site of reaction were assessed for basement membrane, cytoplasmic or lumenal surface association. 125I-labelled antibodies were injected into nude mice bearing LS174T tumour. Per cent injected activity per gram was measured in tumour and normal tissues, 24, 72 and 168 h later. Tissues reacted immunohistochemically for CEA were autoradiographed to assess the relationship of injected antibody to target antigen. Immunohistochemistry showed that A5B7 antibody favours basement membrane aspects of malignant glands; in contrast, EA77 concentrated generally on lumenal surfaces. In vivo localisation showed that per cent inj.act g-1 in tumour for A5B7 reached 36.5% at 24 h. EA77 localised to a lesser extent (9.1% at 24 h), despite a longer circulatory half-life. Autoradiography combined with immunohistochemistry showed A5B7 reacting with antigen close to vasculature after 24 h, slowly penetrating deeper parts of the tumour by 72 h. In contrast, EA77 was confined mainly to fibrovascular stroma, showing little labelling of antigen-positive tumour cells. Localisation differences between A5B7 and EA77 may partly be due to accessibility of epitopes on tumour cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Nakane P. K., Brown W. R. Ultrastructural localization of carcinoembryonic antigen in normal intestine and colon cancer: abnormal distribution of CEA on the surfaces of colon cancer cells. Cancer. 1982 May 15;49(10):2077–2090. doi: 10.1002/1097-0142(19820515)49:10<2077::aid-cncr2820491020>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Begent R. H., Ledermann J. A., Green A. J., Bagshawe K. D., Riggs S. J., Searle F., Keep P. A., Adam T., Dale R. G., Glaser M. G. Antibody distribution and dosimetry in patients receiving radiolabelled antibody therapy for colorectal cancer. Br J Cancer. 1989 Sep;60(3):406–412. doi: 10.1038/bjc.1989.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair S. D., Theodorou N. A., Begent R. H., Dawson P. M., Salmon M., Riggs S., Kelly A., Boxer G., Southall P., Gregory P. Comparison of anti-fetal colonic microvillus and anti-CEA antibodies in peroperative radioimmunolocalisation of colorectal cancer. Br J Cancer. 1990 Jun;61(6):891–894. doi: 10.1038/bjc.1990.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer G. M., Begent R. H., Kelly A. M., Southall P. J., Blair S. B., Theodorou N. A., Dawson P. M., Ledermann J. A. Factors influencing variability of localisation of antibodies to carcinoembryonic antigen (CEA) in patients with colorectal carcinoma--implications for radioimmunotherapy. Br J Cancer. 1992 Jun;65(6):825–831. doi: 10.1038/bjc.1992.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P. A. Heterogeneous expression of cell-surface antigens in normal epithelia and their tumours, revealed by monoclonal antibodies. Br J Cancer. 1985 Feb;51(2):149–160. doi: 10.1038/bjc.1985.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossbard M. L., Press O. W., Appelbaum F. R., Bernstein I. D., Nadler L. M. Monoclonal antibody-based therapies of leukemia and lymphoma. Blood. 1992 Aug 15;80(4):863–878. [PubMed] [Google Scholar]
- Harwood P. J., Britton D. W., Southall P. J., Boxer G. M., Rawlins G., Rogers G. T. Mapping epitope characteristics on carcinoembryonic antigen. Br J Cancer. 1986 Jul;54(1):75–82. doi: 10.1038/bjc.1986.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzlinger D. A., Ojakian G. K. Studies on the development and maintenance of epithelial cell surface polarity with monoclonal antibodies. J Cell Biol. 1984 May;98(5):1777–1787. doi: 10.1083/jcb.98.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humm J. L., Cobb L. M. Nonuniformity of tumor dose in radioimmunotherapy. J Nucl Med. 1990 Jan;31(1):75–83. [PubMed] [Google Scholar]
- Jain R. K. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989 Apr 19;81(8):570–576. doi: 10.1093/jnci/81.8.570. [DOI] [PubMed] [Google Scholar]
- Kyriakos R. J., Shih L. B., Ong G. L., Patel K., Goldenberg D. M., Mattes M. J. The fate of antibodies bound to the surface of tumor cells in vitro. Cancer Res. 1992 Feb 15;52(4):835–842. [PubMed] [Google Scholar]
- Nap M., Hammarström M. L., Börmer O., Hammarström S., Wagener C., Handt S., Schreyer M., Mach J. P., Buchegger F., von Kleist S. Specificity and affinity of monoclonal antibodies against carcinoembryonic antigen. Cancer Res. 1992 Apr 15;52(8):2329–2339. [PubMed] [Google Scholar]
- Nuti M., Teramoto Y. A., Mariani-Costantini R., Hand P. H., Colcher D., Schlom J. A monoclonal antibody (B72.3) defines patterns of distribution of a novel tumor-associated antigen in human mammary carcinoma cell populations. Int J Cancer. 1982 May 15;29(5):539–545. doi: 10.1002/ijc.2910290509. [DOI] [PubMed] [Google Scholar]
- Pedley R. B., Begent R. H., Boden J. A., Boden R., Adam T., Bagshawe K. D. The effect of radiosensitizers on radio-immunotherapy, using 131I-labelled anti-CEA antibodies in a human colonic xenograft model. Int J Cancer. 1991 Feb 20;47(4):597–602. doi: 10.1002/ijc.2910470420. [DOI] [PubMed] [Google Scholar]
- Pedley R. B., Boden J., Keep P. A., Harwood P. J., Green A. J., Rogers G. T. Relationship between tumour size and uptake of radiolabelled anti-CEA in a colon tumour xenograft. Eur J Nucl Med. 1987;13(4):197–202. doi: 10.1007/BF00256491. [DOI] [PubMed] [Google Scholar]
- Pedley R. B., Boxer G. M., Boden J. A., Southall P. J., Begent R. H., Bagshawe K. D., Humm J., Searle F. Preliminary observations on the microdistribution of labelled antibodies in human colonic adenocarcinoma xenografts: relevance to microdosimetry. Br J Cancer. 1990 Feb;61(2):218–220. doi: 10.1038/bjc.1990.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervez S., Epenetos A. A., Mooi W. J., Evans D. J., Rowlinson G., Dhokia B., Krausz T. Localization of monoclonal antibody AUA1 and its F(ab')2 fragments in human tumour xenografts: an autoradiographic and immunohistochemical study. Int J Cancer Suppl. 1988;3:23–29. doi: 10.1002/ijc.2910410806. [DOI] [PubMed] [Google Scholar]
- Pervez S., Kirkland S. C., Epenetos A. A., Mooi W. J., Evans D. J., Krausz T. Effect of polarity and differentiation on antibody localization in multicellular tumour spheroid and xenograft models and its potential importance for in vivo immunotargeting. Int J Cancer. 1989 Nov 15;44(5):940–947. doi: 10.1002/ijc.2910440532. [DOI] [PubMed] [Google Scholar]
- Poznansky M. J., Juliano R. L. Biological approaches to the controlled delivery of drugs: a critical review. Pharmacol Rev. 1984 Dec;36(4):277–336. [PubMed] [Google Scholar]
- Shockley T. R., Lin K., Nagy J. A., Tompkins R. G., Dvorak H. F., Yarmush M. L. Penetration of tumor tissue by antibodies and other immunoproteins. Ann N Y Acad Sci. 1991;618:367–382. doi: 10.1111/j.1749-6632.1991.tb27257.x. [DOI] [PubMed] [Google Scholar]
- Southall P. J., Boxer G. M., Bagshawe K. D., Hole N., Bromley M., Stern P. L. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer. 1990 Jan;61(1):89–95. doi: 10.1038/bjc.1990.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steis R. G., Carrasquillo J. A., McCabe R., Bookman M. A., Reynolds J. C., Larson S. M., Smith J. W., 2nd, Clark J. W., Dailey V., Del Vecchio S. Toxicity, immunogenicity, and tumor radioimmunodetecting ability of two human monoclonal antibodies in patients with metastatic colorectal carcinoma. J Clin Oncol. 1990 Mar;8(3):476–490. doi: 10.1200/JCO.1990.8.3.476. [DOI] [PubMed] [Google Scholar]
- Tom B. H., Rutzky L. P., Jakstys M. M., Oyasu R., Kaye C. I., Kahan B. D. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976 Mar;12(3):180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- Welt S., Divgi C. R., Real F. X., Yeh S. D., Garin-Chesa P., Finstad C. L., Sakamoto J., Cohen A., Sigurdson E. R., Kemeny N. Quantitative analysis of antibody localization in human metastatic colon cancer: a phase I study of monoclonal antibody A33. J Clin Oncol. 1990 Nov;8(11):1894–1906. doi: 10.1200/JCO.1990.8.11.1894. [DOI] [PubMed] [Google Scholar]
- Yokota T., Milenic D. E., Whitlow M., Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992 Jun 15;52(12):3402–3408. [PubMed] [Google Scholar]