Abstract
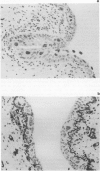
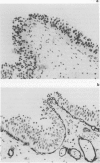
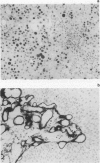
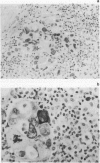
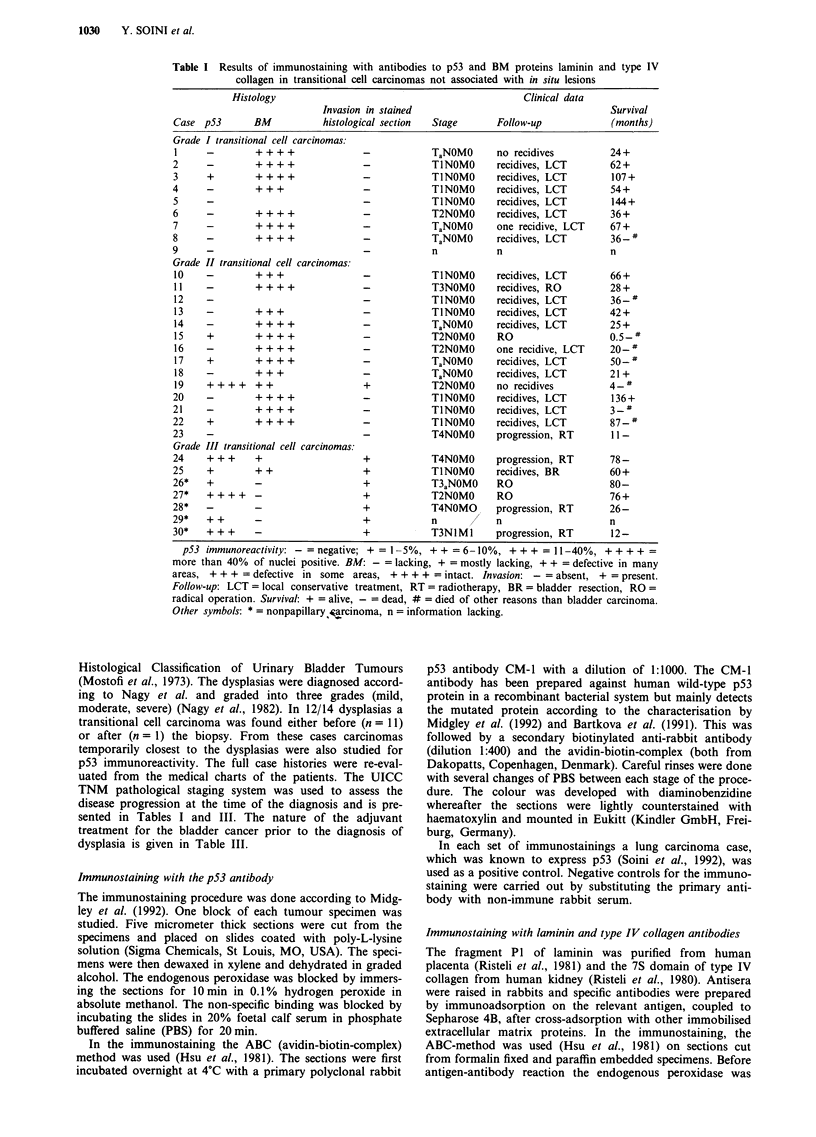
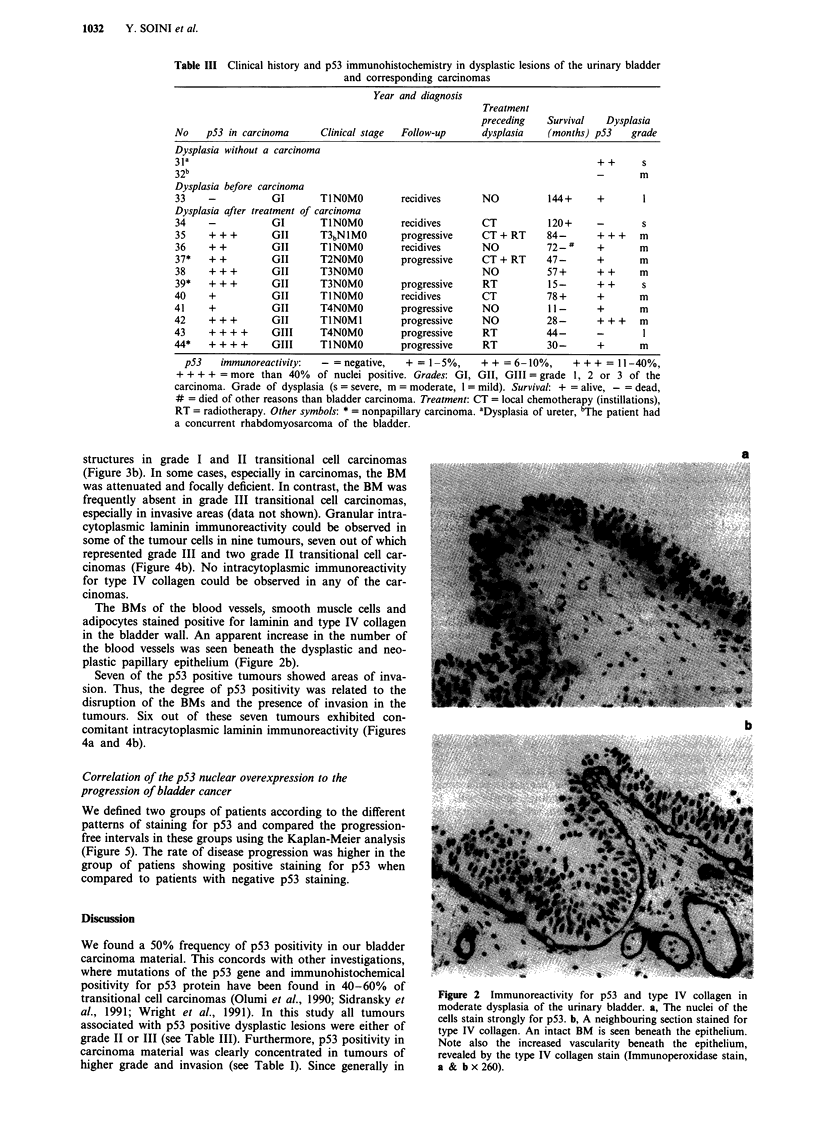
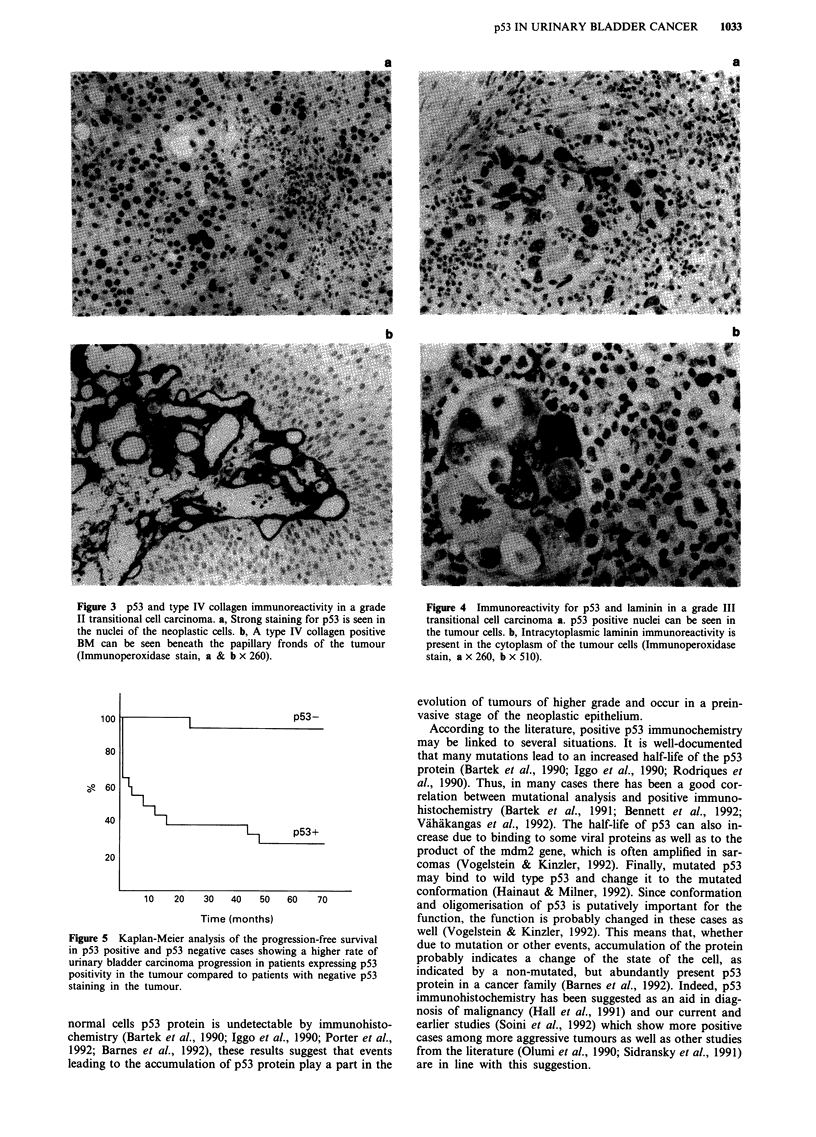
Immunohistochemically detectable p53 protein using a polyclonal antibody (CM-1) was studied in 42 carcinomas of which 11 were grade I, 22 grade II and nine grade III carcinomas. Additionally 14 urothelial dysplasias were studied. In 11 of these a diagnosis of transitional cell carcinoma was established before and in one after the dysplasia diagnosis. Twenty-one out of 42 (50%) cases of transitional cell carcinoma were positive for the p53 protein. Eleven out of 14 (78%) dysplasias and 10/12 (83%) related carcinomas were p53 positive. One out of 11 grade I (9%), 12/22 grade II (55%) and 8/9 grade III (89%) tumours showed positivity for p53. There were significantly more p53 positive cases in grade II-III tumours than in grade I tumours (P = 0.004). There were significantly more p53 positive cases in stage T2-T4 tumours than in stage T1 tumours (P = 0.035). In only one case among the 11 dysplastic lesions following the treatment of a carcinoma the dysplastic lesion was p53 negative while the preceding carcinoma was p53 positive. All dysplasias and 28 carcinomas were also immunostained for laminin and type IV collagen to evaluate the continuity of basement membranes (BMs). Clearly disrupted BMs were observed only in grade III carcinomas. These cases showed the most p53 immunopositivity. The results show a strong association of p53 staining between dysplasias and transitional cell carcinomas of the urinary bladder indicating that these lesions might share similar p53 changes. The correlation to grade, clinical stage and to disrupted BM suggests that p53 mutations may be associated with the evolution of aggressive growth characteristics in transitional cell carcinomas or, alternatively, that p53 positive tumours of a more aggressive type from the start. Whether p53 staining can be used as an adjunct in the assessment and follow-up of epithelial changes of patients treated for a p53 positive bladder carcinoma deserves to be studied.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Barnes D. M., Hanby A. M., Gillett C. E., Mohammed S., Hodgson S., Bobrow L. G., Leigh I. M., Purkis T., MacGeoch C., Spurr N. K. Abnormal expression of wild type p53 protein in normal cells of a cancer family patient. Lancet. 1992 Aug 1;340(8814):259–263. doi: 10.1016/0140-6736(92)92354-i. [DOI] [PubMed] [Google Scholar]
- Bennett W. P., Hollstein M. C., He A., Zhu S. M., Resau J. H., Trump B. F., Metcalf R. A., Welsh J. A., Midgley C., Lane D. P. Archival analysis of p53 genetic and protein alterations in Chinese esophageal cancer. Oncogene. 1991 Oct;6(10):1779–1784. [PubMed] [Google Scholar]
- Bischoff J. R., Friedman P. N., Marshak D. R., Prives C., Beach D. Human p53 is phosphorylated by p60-cdc2 and cyclin B-cdc2. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4766–4770. doi: 10.1073/pnas.87.12.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman F. T., Havenith M., Cleutjens J. P. Basement membranes in cancer. Ultrastruct Pathol. 1985;8(4):291–304. doi: 10.3109/01913128509141519. [DOI] [PubMed] [Google Scholar]
- Bártek J., Bártková J., Vojtesek B., Stasková Z., Lukás J., Rejthar A., Kovarík J., Midgley C. A., Gannon J. V., Lane D. P. Aberrant expression of the p53 oncoprotein is a common feature of a wide spectrum of human malignancies. Oncogene. 1991 Sep;6(9):1699–1703. [PubMed] [Google Scholar]
- Bártková J., Bártek J., Lukás J., Vojtesek B., Stasková Z., Rejthar A., Kovarík J., Midgley C. A., Lane D. P. p53 protein alterations in human testicular cancer including pre-invasive intratubular germ-cell neoplasia. Int J Cancer. 1991 Sep 9;49(2):196–202. doi: 10.1002/ijc.2910490209. [DOI] [PubMed] [Google Scholar]
- Casson A. G., Mukhopadhyay T., Cleary K. R., Ro J. Y., Levin B., Roth J. A. p53 gene mutations in Barrett's epithelium and esophageal cancer. Cancer Res. 1991 Aug 15;51(16):4495–4499. [PubMed] [Google Scholar]
- Chiba I., Takahashi T., Nau M. M., D'Amico D., Curiel D. T., Mitsudomi T., Buchhagen D. L., Carbone D., Piantadosi S., Koga H. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung Cancer Study Group. Oncogene. 1990 Oct;5(10):1603–1610. [PubMed] [Google Scholar]
- Deppert W., Buschhausen-Denker G., Patschinsky T., Steinmeyer K. Cell cycle control of p53 in normal (3T3) and chemically transformed (Meth A) mouse cells. II. Requirement for cell cycle progression. Oncogene. 1990 Nov;5(11):1701–1706. [PubMed] [Google Scholar]
- Ekblom P., Miettinen M., Rapola J., Foidart J. M. Demonstration of laminin, a basement membrane glycoprotein, in routinely processed formalin-fixed human tissues. Histochemistry. 1982;75(3):301–307. doi: 10.1007/BF00496733. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer G., Bargonetti J., Zhu H., Friedman P., Prywes R., Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992 Jul 2;358(6381):83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Gusterson B. A., Anbazhagan R., Warren W., Midgely C., Lane D. P., O'Hare M., Stamps A., Carter R., Jayatilake H. Expression of p53 in premalignant and malignant squamous epithelium. Oncogene. 1991 Oct;6(10):1785–1789. [PubMed] [Google Scholar]
- Hainaut P., Milner J. Interaction of heat-shock protein 70 with p53 translated in vitro: evidence for interaction with dimeric p53 and for a role in the regulation of p53 conformation. EMBO J. 1992 Oct;11(10):3513–3520. doi: 10.1002/j.1460-2075.1992.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Rodel J., Peled A., Oren M. Frequent p53 mutations in chemically induced murine fibrosarcoma. Oncogene. 1991 Sep;6(9):1593–1600. [PubMed] [Google Scholar]
- Hall P. A., Ray A., Lemoine N. R., Midgley C. A., Krausz T., Lane D. P. p53 immunostaining as a marker of malignant disease in diagnostic cytopathology. Lancet. 1991 Aug 24;338(8765):513–513. doi: 10.1016/0140-6736(91)90586-e. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Ishikawa J., Xu H. J., Hu S. X., Yandell D. W., Maeda S., Kamidono S., Benedict W. F., Takahashi R. Inactivation of the retinoblastoma gene in human bladder and renal cell carcinomas. Cancer Res. 1991 Oct 15;51(20):5736–5743. [PubMed] [Google Scholar]
- Kakizoe T., Matumoto K., Nishio Y., Ohtani M., Kishi K. Significance of carcinoma in situ and dysplasia in association with bladder cancer. J Urol. 1985 Mar;133(3):395–398. doi: 10.1016/s0022-5347(17)48994-4. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The p53 tumour suppressor gene and product. Cancer Surv. 1992;12:59–79. [PubMed] [Google Scholar]
- Lu X., Park S. H., Thompson T. C., Lane D. P. Ras-induced hyperplasia occurs with mutation of p53, but activated ras and myc together can induce carcinoma without p53 mutation. Cell. 1992 Jul 10;70(1):153–161. doi: 10.1016/0092-8674(92)90541-j. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- Mazars R., Pujol P., Maudelonde T., Jeanteur P., Theillet C. p53 mutations in ovarian cancer: a late event? Oncogene. 1991 Sep;6(9):1685–1690. [PubMed] [Google Scholar]
- Mercer W. E., Avignolo C., Baserga R. Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol Cell Biol. 1984 Feb;4(2):276–281. doi: 10.1128/mcb.4.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley C. A., Fisher C. J., Bártek J., Vojtesek B., Lane D., Barnes D. M. Analysis of p53 expression in human tumours: an antibody raised against human p53 expressed in Escherichia coli. J Cell Sci. 1992 Jan;101(Pt 1):183–189. doi: 10.1242/jcs.101.1.183. [DOI] [PubMed] [Google Scholar]
- Milner J. The role of p53 in the normal control of cell proliferation. Curr Opin Cell Biol. 1991 Apr;3(2):282–286. doi: 10.1016/0955-0674(91)90153-p. [DOI] [PubMed] [Google Scholar]
- Nagy G. K., Frable W. J., Murphy W. M. Classification of premalignant urothelial abnormalities. A Delphi study of the National Bladder Cancer Collaborative Group A. Pathol Annu. 1982;17(Pt 1):219–233. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Nuorva K., Soini Y., Kamel D., Autio-Harmainen H., Risteli L., Risteli J., Vähäkangas K., Päkkö P. Concurrent p53 expression in bronchial dysplasias and squamous cell lung carcinomas. Am J Pathol. 1993 Mar;142(3):725–732. [PMC free article] [PubMed] [Google Scholar]
- Ohtani M., Kakizoe T., Nishio Y., Sato S., Sugimura T., Fukushima S., Niijima T. Sequential changes of mouse bladder epithelium during induction of invasive carcinomas by N-butyl-N-(4-hydroxybutyl)nitrosamine. Cancer Res. 1986 Apr;46(4 Pt 2):2001–2004. [PubMed] [Google Scholar]
- Olumi A. F., Tsai Y. C., Nichols P. W., Skinner D. G., Cain D. R., Bender L. I., Jones P. A. Allelic loss of chromosome 17p distinguishes high grade from low grade transitional cell carcinomas of the bladder. Cancer Res. 1990 Nov 1;50(21):7081–7083. [PubMed] [Google Scholar]
- Porter P. L., Gown A. M., Kramp S. G., Coltrera M. D. Widespread p53 overexpression in human malignant tumors. An immunohistochemical study using methacarn-fixed, embedded tissue. Am J Pathol. 1992 Jan;140(1):145–153. [PMC free article] [PubMed] [Google Scholar]
- Purdie C. A., O'Grady J., Piris J., Wyllie A. H., Bird C. C. p53 expression in colorectal tumors. Am J Pathol. 1991 Apr;138(4):807–813. [PMC free article] [PubMed] [Google Scholar]
- Risinger J. I., Dent G. A., Ignar-Trowbridge D., McLachlan J. A., Tsao M. S., Senterman M., Boyd J. p53 gene mutations in human endometrial carcinoma. Mol Carcinog. 1992;5(4):250–253. doi: 10.1002/mc.2940050403. [DOI] [PubMed] [Google Scholar]
- Risteli J., Bächinger H. P., Engel J., Furthmayr H., Timpl R. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem. 1980;108(1):239–250. doi: 10.1111/j.1432-1033.1980.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Risteli L., Timpl R. Isolation and characterization of pepsin fragments of laminin from human placental and renal basement membranes. Biochem J. 1981 Mar 1;193(3):749–755. doi: 10.1042/bj1930749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Schapers R. F., Pauwels R. P., Havenith M. G., Smeets A. W., van den Brandt P. A., Bosman F. T. Prognostic significance of type IV collagen and laminin immunoreactivity in urothelial carcinomas of the bladder. Cancer. 1990 Dec 15;66(12):2583–2588. doi: 10.1002/1097-0142(19901215)66:12<2583::aid-cncr2820661222>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Soini Y., Päkkö P., Nuorva K., Kamel D., Lane D. P., Vähäkangas K. Comparative analysis of p53 protein immunoreactivity in prostatic, lung and breast carcinomas. Virchows Arch A Pathol Anat Histopathol. 1992;421(3):223–228. doi: 10.1007/BF01611179. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Maacke H., Deppert W. Cell cycle control by p53 in normal (3T3) and chemically transformed (Meth A) mouse cells. I. Regulation of p53 expression. Oncogene. 1990 Nov;5(11):1691–1699. [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Vähäkangas K. H., Samet J. M., Metcalf R. A., Welsh J. A., Bennett W. P., Lane D. P., Harris C. C. Mutations of p53 and ras genes in radon-associated lung cancer from uranium miners. Lancet. 1992 Mar 7;339(8793):576–580. doi: 10.1016/0140-6736(92)90866-2. [DOI] [PubMed] [Google Scholar]
- Wolf H., Olsen P. R., Højgaard K. Urothelial dysplasia concomitant with bladder tumours: a determinant for future new occurrences in patients treated by full-course radiotherapy. Lancet. 1985 May 4;1(8436):1005–1008. doi: 10.1016/s0140-6736(85)91612-5. [DOI] [PubMed] [Google Scholar]
- Wright C., Mellon K., Johnston P., Lane D. P., Harris A. L., Horne C. H., Neal D. E. Expression of mutant p53, c-erbB-2 and the epidermal growth factor receptor in transitional cell carcinoma of the human urinary bladder. Br J Cancer. 1991 Jun;63(6):967–970. doi: 10.1038/bjc.1991.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. A., Lemoine N. R., Goretzki P. E., Wyllie F. S., Bond J., Hughes C., Röher H. D., Williams E. D., Wynford-Thomas D. Mutation of the p53 gene in a differentiated human thyroid carcinoma cell line, but not in primary thyroid tumours. Oncogene. 1991 Sep;6(9):1693–1697. [PubMed] [Google Scholar]