Abstract
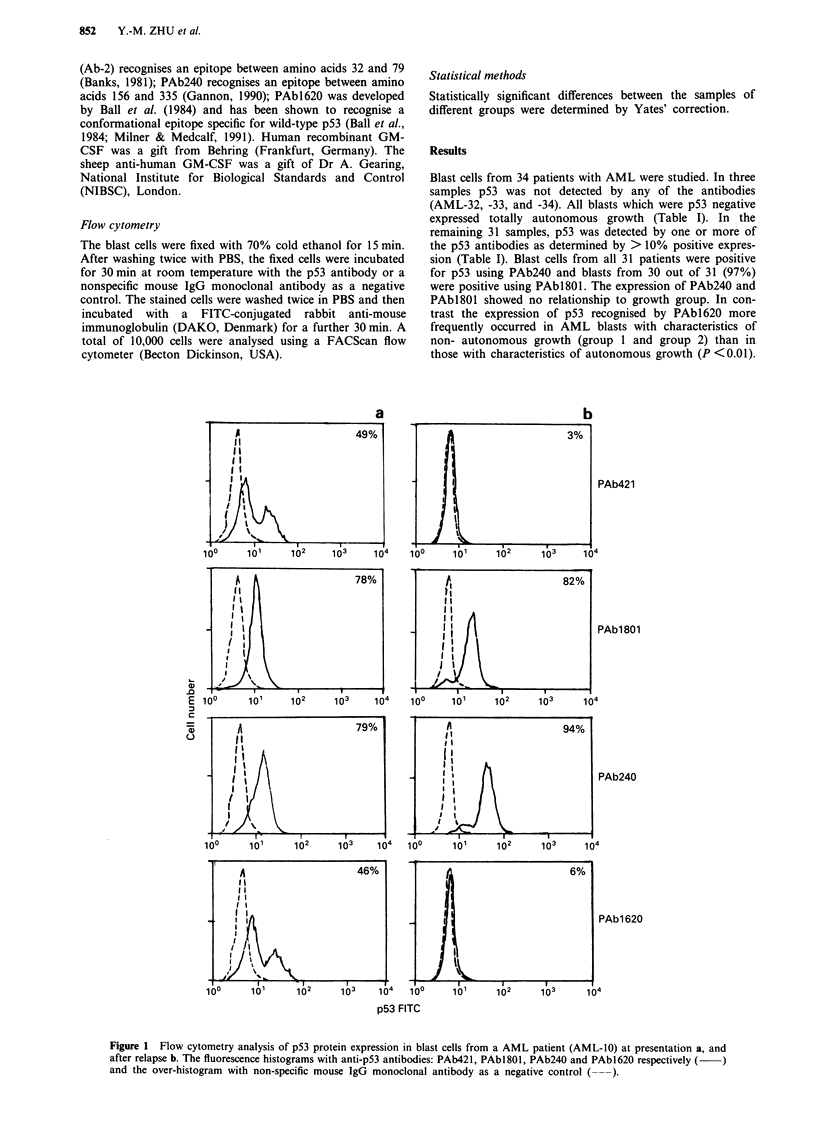
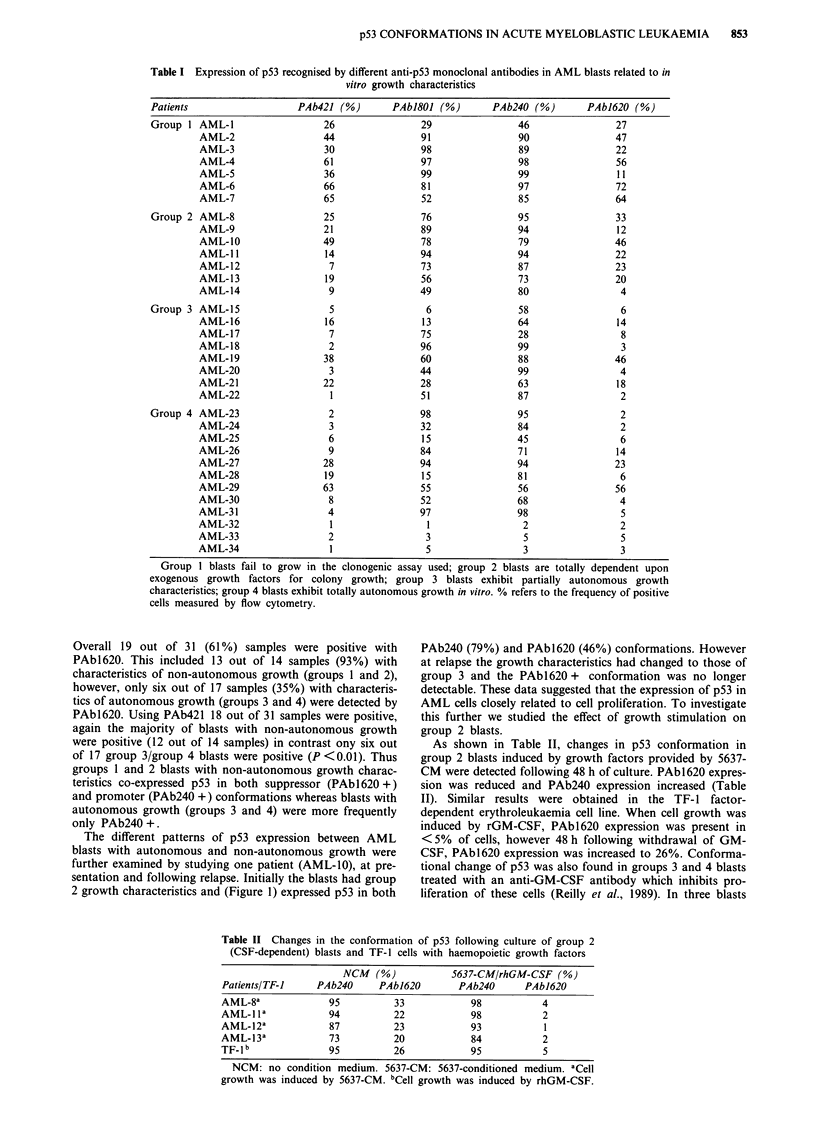
Expression of the wild-type p53 gene has an important role in cell differentiation, maturation and apoptosis. Mutation of the p53 gene is associated with tumour development and mutant p53 can promote cell proliferation. Recently wild-type p53 has been demonstrated to exist in two conformational variants: one acting as a suppressor (PAb240-/PAb1620+) and one as a promoter (PAb240+/PAb1620-) of cell proliferation. We have analysed the expression of p53 by flow cytometry in blast cells from 34 patients with acute myeloblastic leukaemia in relationship to the proliferation characteristics of these cells in a clonogenic assay. Blasts from three out of 34 patients did not express p53 using the antibodies: PAb421, PAb1801, PAb240 and PAb1620. The remaining 31 samples expressed p53 detected by PAb240 which recognises mutant p53 and is predicted to recognise wild-type p53 in the promoter conformation. Blasts from 19 out of 31 cells which expressed PAb240 co-expressed PAb1620, expression of PAb1620 was associated with non-autonomous growth in vitro. In contrast, the majority of blasts with the p53 phenotype of PAb240+/PAb1620- or which lacked p53 expression exhibited autonomous growth characteristics in vitro. Furthermore expression of PAb1620 in blasts with autonomous growth cells could be detected following growth inhibition using monoclonal antibodies against autocrine growth factors. Our data demonstrate that in AML cells, p53 conformation is related to the growth characteristics of the cells and is regulated by either exogenous or autocrine haematopoietic growth factors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball R. K., Siegl B., Quellhorst S., Brandner G., Braun D. G. Monoclonal antibodies against simian virus 40 nuclear large T tumour antigen: epitope mapping, papova virus cross-reaction and cell surface staining. EMBO J. 1984 Jul;3(7):1485–1491. doi: 10.1002/j.1460-2075.1984.tb02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks L., Matlashewski G., Crawford L. Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986 Sep 15;159(3):529–534. doi: 10.1111/j.1432-1033.1986.tb09919.x. [DOI] [PubMed] [Google Scholar]
- Bradbury D., Bowen G., Kozlowski R., Reilly I., Russell N. Endogenous interleukin-1 can regulate the autonomous growth of the blast cells of acute myeloblastic leukemia by inducing autocrine secretion of GM-CSF. Leukemia. 1990 Jan;4(1):44–47. [PubMed] [Google Scholar]
- Danova M., Giordano M., Mazzini G., Riccardi A. Expression of p53 protein during the cell cycle measured by flow cytometry in human leukemia. Leuk Res. 1990;14(5):417–422. doi: 10.1016/0145-2126(90)90027-7. [DOI] [PubMed] [Google Scholar]
- Fenaux P., Preudhomme C., Quiquandon I., Jonveaux P., Laï J. L., Vanrumbeke M., Loucheux-Lefebvre M. H., Bauters F., Berger R., Kerckaert J. P. Mutations of the P53 gene in acute myeloid leukaemia. Br J Haematol. 1992 Feb;80(2):178–183. doi: 10.1111/j.1365-2141.1992.tb08897.x. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M., Danova M., Riccardi A., Mazzini G. Simultaneous detection of cellular ras p21 oncogene product and DNA content by two-parameter flow cytometry. Anticancer Res. 1989 May-Jun;9(3):799–803. [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Zhang W., Deisseroth A. B. P53 gene mutations in acute myelogenous leukaemia. Br J Haematol. 1992 Aug;81(4):489–494. doi: 10.1111/j.1365-2141.1992.tb02979.x. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Tange T., Terasawa T., Chiba S., Kuwaki T., Miyagawa K., Piao Y. F., Miyazono K., Urabe A., Takaku F. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989 Aug;140(2):323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Lanza F., Bi S., Goldman J. M. Similarity of p53 expression by CD34+ cells in chronic myeloid leukemia and normal progenitors detected by flow cytometry. Blood. 1992 Oct 1;80(7):1857–1858. [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Milner J. A conformation hypothesis for the suppressor and promoter functions of p53 in cell growth control and in cancer. Proc Biol Sci. 1991 Aug 22;245(1313):139–145. doi: 10.1098/rspb.1991.0100. [DOI] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A. Cotranslation of activated mutant p53 with wild type drives the wild-type p53 protein into the mutant conformation. Cell. 1991 May 31;65(5):765–774. doi: 10.1016/0092-8674(91)90384-b. [DOI] [PubMed] [Google Scholar]
- Milner J., Watson J. V. Addition of fresh medium induces cell cycle and conformation changes in p53, a tumour suppressor protein. Oncogene. 1990 Nov;5(11):1683–1690. [PubMed] [Google Scholar]
- Reilly I. A., Kozlowski R., Russell N. H. Heterogeneous mechanisms of autocrine growth of AML blasts. Br J Haematol. 1989 Jul;72(3):363–369. doi: 10.1111/j.1365-2141.1989.tb07717.x. [DOI] [PubMed] [Google Scholar]
- Rivas C. I., Wisniewski D., Strife A., Perez A., Lambek C., Bruno S., Darzynkiewicz Z., Clarkson B. Constitutive expression of p53 protein in enriched normal human marrow blast cell populations. Blood. 1992 Apr 15;79(8):1982–1986. [PubMed] [Google Scholar]
- Sugimoto K., Toyoshima H., Sakai R., Miyagawa K., Hagiwara K., Ishikawa F., Takaku F., Yazaki Y., Hirai H. Frequent mutations in the p53 gene in human myeloid leukemia cell lines. Blood. 1992 May 1;79(9):2378–2383. [PubMed] [Google Scholar]
- Ullrich S. J., Mercer W. E., Appella E. Human wild-type p53 adopts a unique conformational and phosphorylation state in vivo during growth arrest of glioblastoma cells. Oncogene. 1992 Aug;7(8):1635–1643. [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Zhang W., Hu G., Estey E., Hester J., Deisseroth A. Altered conformation of the p53 protein in myeloid leukemia cells and mitogen-stimulated normal blood cells. Oncogene. 1992 Aug;7(8):1645–1647. [PubMed] [Google Scholar]


