Abstract
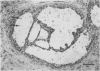
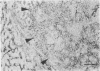
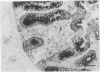
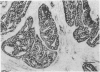
CD59 (protectin) and CD46 (membrane cofactor protein, MCP) are membrane-bound complement regulator proteins which inhibit complement-mediated cytolysis of autologous cells. CD59, a phosphatidyl-inositol-anchored glycoprotein, inhibits the formation of the terminal membrane attack complex (MAC) of complement and was found to be a second ligand for CD2 contributing to T-cell activation. In 20 colorectal normal mucosa samples, in ten adenomas, 71 carcinomas and in ten liver metastases derived thereof, CD59 was inconsistently expressed in the epithelial compartment. In carcinomas CD59 expression in the whole neoplastic compartment was more often found in well- and moderately differentiated tumours. By contrast, focal expression or even complete lack of CD59 was more often found in poorly differentiated tumours (P = 0.021). In addition, carcinomas without metastases at the time of operation (Dukes A/B) more often expressed CD59 in the entire neoplastic population compared to those carcinomas which had already metastasised (P = 0.018). There was no correlation between the mode of CD59 expression in colorectal carcinomas and the tumour type or location. CD46 has C3b/C4b binding and factor-I dependent cofactor activity and is broadly expressed in various cells and tissues. In the epithelial compartment of normal colorectal mucosa, of all adenomas, carcinomas and their liver metastases, CD46 was expressed throughout the epithelial compartment. Since CD46 was consistently expressed in colorectal carcinomas the low expression or even lack of CD59 in a subset of tumours might not lead to critical complement-mediated attack of CD59-negative tumour cells. Regarding CD59 as a natural T-cell ligand involved in cognate T-cell-target-cell interaction, however, loss of CD59 might well be a selection advantage, provided that tumour antigen-mediated T-cell toxicity in colorectal carcinoma exists.
Full text
PDF


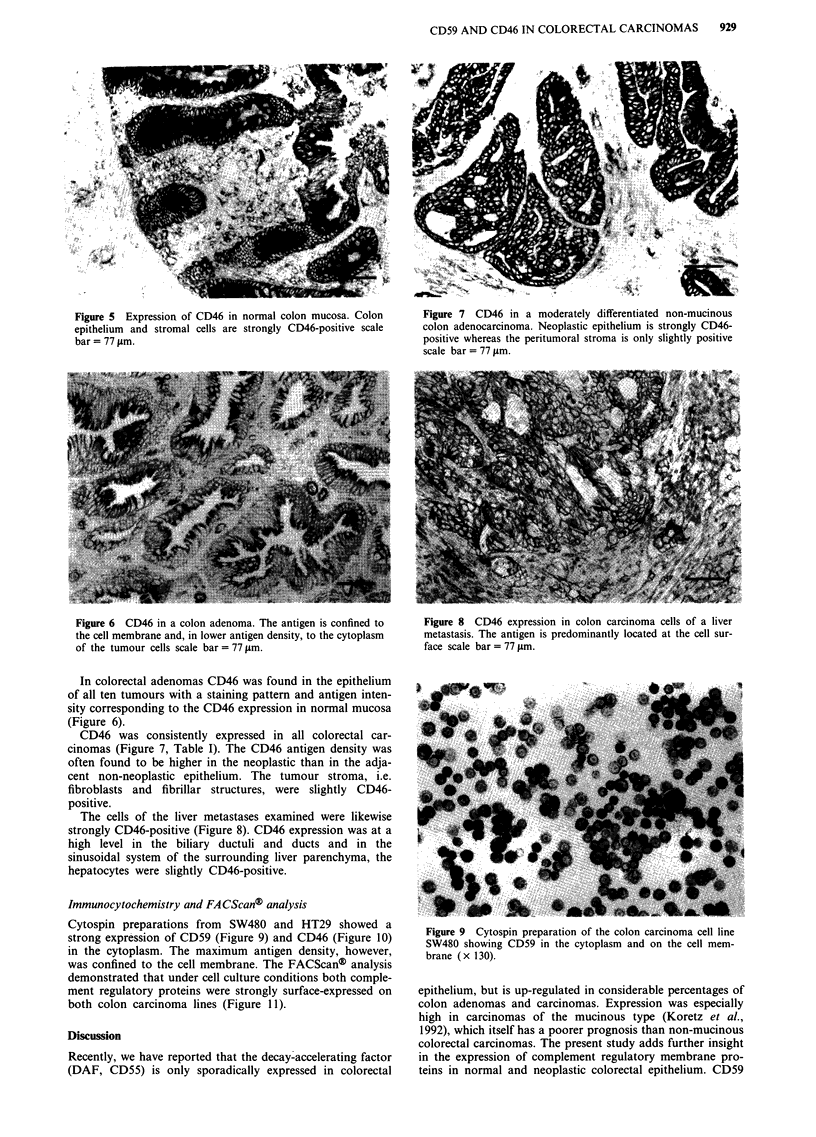
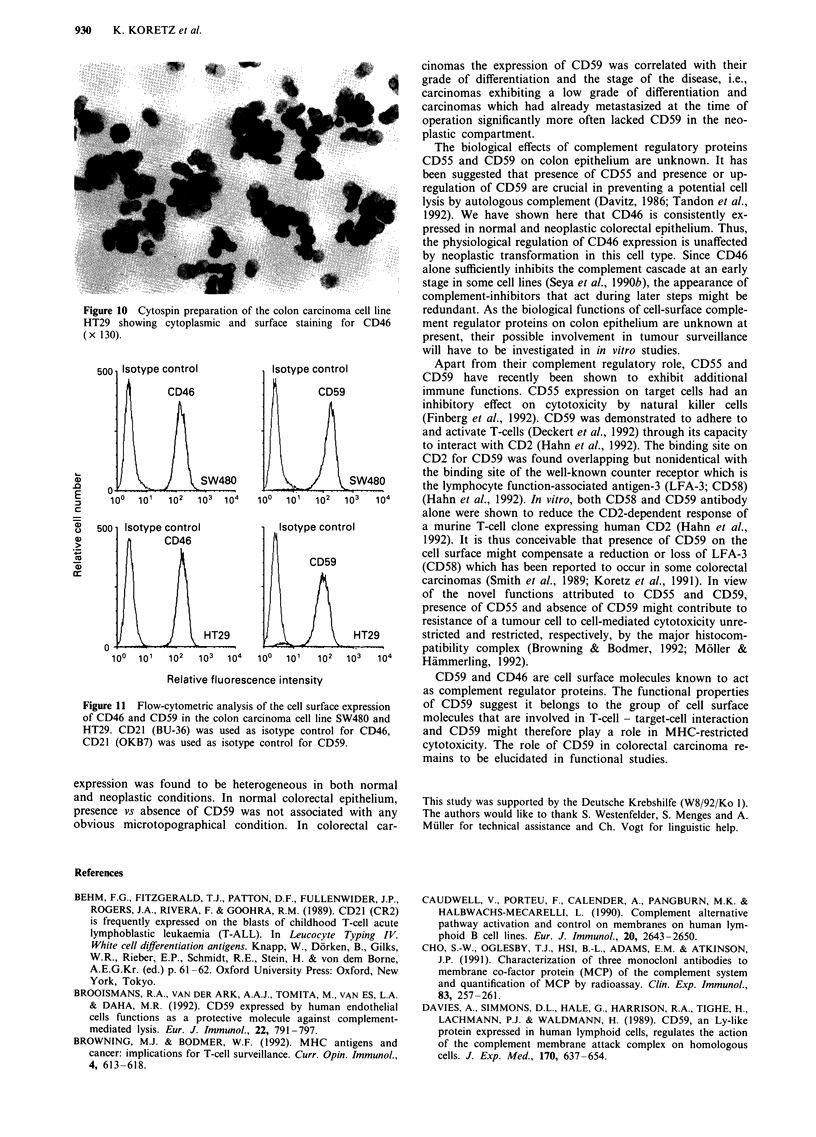

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooimans R. A., Van der Ark A. A., Tomita M., Van Es L. A., Daha M. R. CD59 expressed by human endothelial cells functions as a protective molecule against complement-mediated lysis. Eur J Immunol. 1992 Mar;22(3):791–797. doi: 10.1002/eji.1830220324. [DOI] [PubMed] [Google Scholar]
- Browning M. J., Bodmer W. F. MHC antigens and cancer: implications for T-cell surveillance. Curr Opin Immunol. 1992 Oct;4(5):613–618. doi: 10.1016/0952-7915(92)90036-e. [DOI] [PubMed] [Google Scholar]
- Caudwell V., Porteu F., Calender A., Pangburn M. K., Halbwachs-Mecarelli L. Complement alternative pathway activation and control on membranes of human lymphoid B cell lines. Eur J Immunol. 1990 Dec;20(12):2643–2650. doi: 10.1002/eji.1830201218. [DOI] [PubMed] [Google Scholar]
- Cho S. W., Oglesby T. J., Hsi B. L., Adams E. M., Atkinson J. P. Characterization of three monoclonal antibodies to membrane co-factor protein (MCP) of the complement system and quantification of MCP by radioassay. Clin Exp Immunol. 1991 Feb;83(2):257–261. doi: 10.1111/j.1365-2249.1991.tb05624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUKES C. E., BUSSEY H. J. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958 Sep;12(3):309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davitz M. A. Decay-accelerating factor (DAF): a review of its function and structure. Acta Med Scand Suppl. 1987;715:111–121. doi: 10.1111/j.0954-6820.1987.tb09911.x. [DOI] [PubMed] [Google Scholar]
- Deckert M., Kubar J., Bernard A. CD58 and CD59 molecules exhibit potentializing effects in T cell adhesion and activation. J Immunol. 1992 Feb 1;148(3):672–677. [PubMed] [Google Scholar]
- Finberg R. W., White W., Nicholson-Weller A. Decay-accelerating factor expression on either effector or target cells inhibits cytotoxicity by human natural killer cells. J Immunol. 1992 Sep 15;149(6):2055–2060. [PubMed] [Google Scholar]
- Hahn W. C., Menu E., Bothwell A. L., Sims P. J., Bierer B. E. Overlapping but nonidentical binding sites on CD2 for CD58 and a second ligand CD59. Science. 1992 Jun 26;256(5065):1805–1807. doi: 10.1126/science.1377404. [DOI] [PubMed] [Google Scholar]
- Kinoshita T. Biology of complement: the overture. Immunol Today. 1991 Sep;12(9):291–295. doi: 10.1016/0167-5699(91)90001-A. [DOI] [PubMed] [Google Scholar]
- Koretz K., Brüderlein S., Henne C., Möller P. Decay-accelerating factor (DAF, CD55) in normal colorectal mucosa, adenomas and carcinomas. Br J Cancer. 1992 Nov;66(5):810–814. doi: 10.1038/bjc.1992.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretz K., Schlag P., Möller P. Sporadic loss of leucocyte-function-associated antigen-3 (LFA-3) in colorectal carcinomas. Virchows Arch A Pathol Anat Histopathol. 1991;419(5):389–394. doi: 10.1007/BF01605072. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J. The control of homologous lysis. Immunol Today. 1991 Sep;12(9):312–315. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Post T. W., Atkinson J. P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor and membrane cofactor protein. Curr Top Microbiol Immunol. 1990;153:123–145. doi: 10.1007/978-3-642-74977-3_7. [DOI] [PubMed] [Google Scholar]
- McNearney T., Ballard L., Seya T., Atkinson J. P. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989 Aug;84(2):538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meri S., Morgan B. P., Davies A., Daniels R. H., Olavesen M. G., Waldmann H., Lachmann P. J. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990 Sep;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Meri S., Waldmann H., Lachmann P. J. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991 Nov;65(5):532–537. [PubMed] [Google Scholar]
- Möller P., Hämmerling G. J. The role of surface HLA-A,B,C molecules in tumour immunity. Cancer Surv. 1992;13:101–127. [PubMed] [Google Scholar]
- Pesando J. M., Stucki M., Hoffman P. Altered expression of surface antigens with appearance of HLA class II molecules on a malignant human B-cell line. Hum Immunol. 1987 Aug;19(4):235–243. doi: 10.1016/0198-8859(87)90041-3. [DOI] [PubMed] [Google Scholar]
- Ratnoff W. D., Knez J. J., Prince G. M., Okada H., Lachmann P. J., Medof M. E. Structural properties of the glycoplasmanylinositol anchor phospholipid of the complement membrane attack complex inhibitor CD59. Clin Exp Immunol. 1992 Mar;87(3):415–421. doi: 10.1111/j.1365-2249.1992.tb03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., Taylor C. T., Melling G. C., Kingsland C. R., Johnson P. M. Expression of the CD46 antigen, and absence of class I MHC antigen, on the human oocyte and preimplantation blastocyst. Immunology. 1992 Jan;75(1):202–205. [PMC free article] [PubMed] [Google Scholar]
- Rooney I. A., Davies A., Griffiths D., Williams J. D., Davies M., Meri S., Lachmann P. J., Morgan B. P. The complement-inhibiting protein, protectin (CD59 antigen), is present and functionally active on glomerular epithelial cells. Clin Exp Immunol. 1991 Feb;83(2):251–256. doi: 10.1111/j.1365-2249.1991.tb05623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney I. A., Davies A., Morgan B. P. Membrane attack complex (MAC)-mediated damage to spermatozoa: protection of the cells by the presence on their membranes of MAC inhibitory proteins. Immunology. 1992 Mar;75(3):499–506. [PMC free article] [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol. 1990 Jul 1;145(1):238–245. [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Sugita Y., Akedo H. Complement-mediated tumor cell damage induced by antibodies against membrane cofactor protein (MCP, CD46). J Exp Med. 1990 Dec 1;172(6):1673–1680. doi: 10.1084/jem.172.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Okada M., Matsumoto M., Hong K. S., Kinoshita T., Atkinson J. P. Preferential inactivation of the C5 convertase of the alternative complement pathway by factor I and membrane cofactor protein (MCP). Mol Immunol. 1991 Oct;28(10):1137–1147. doi: 10.1016/0161-5890(91)90029-j. [DOI] [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. E., Marsh S. G., Bodmer J. G., Gelsthorpe K., Bodmer W. F. Loss of HLA-A,B,C allele products and lymphocyte function-associated antigen 3 in colorectal neoplasia. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5557–5561. doi: 10.1073/pnas.86.14.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Hilgert I., Kristofová H., Brown R., Low M. G., Horejsí V. Characterization of a broadly expressed human leucocyte surface antigen MEM-43 anchored in membrane through phosphatidylinositol. Mol Immunol. 1989 Feb;26(2):153–161. doi: 10.1016/0161-5890(89)90097-7. [DOI] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Tandon N., Morgan B. P., Weetman A. P. Expression and function of membrane attack complex inhibitory proteins on thyroid follicular cells. Immunology. 1992 Feb;75(2):372–377. [PMC free article] [PubMed] [Google Scholar]
- Venneker G. T., Asghar S. S. CD59: a molecule involved in antigen presentation as well as downregulation of membrane attack complex. Exp Clin Immunogenet. 1992;9(1):33–47. [PubMed] [Google Scholar]