Abstract
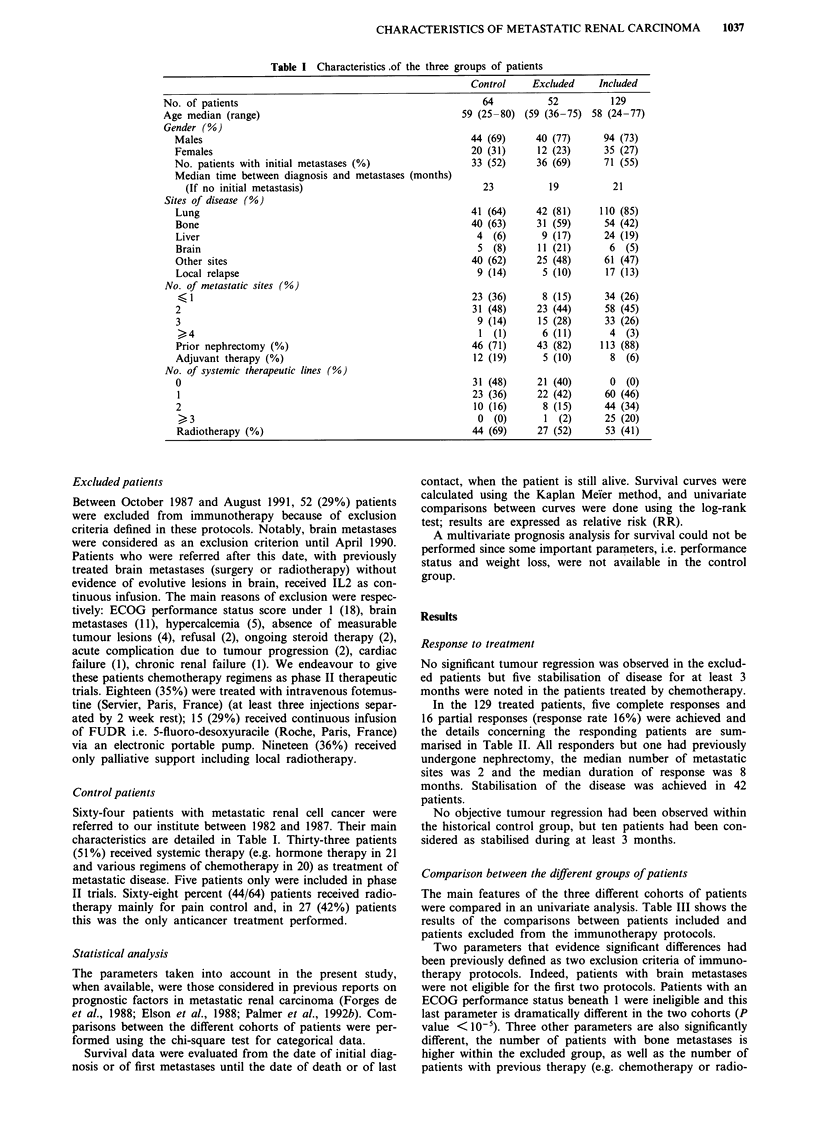
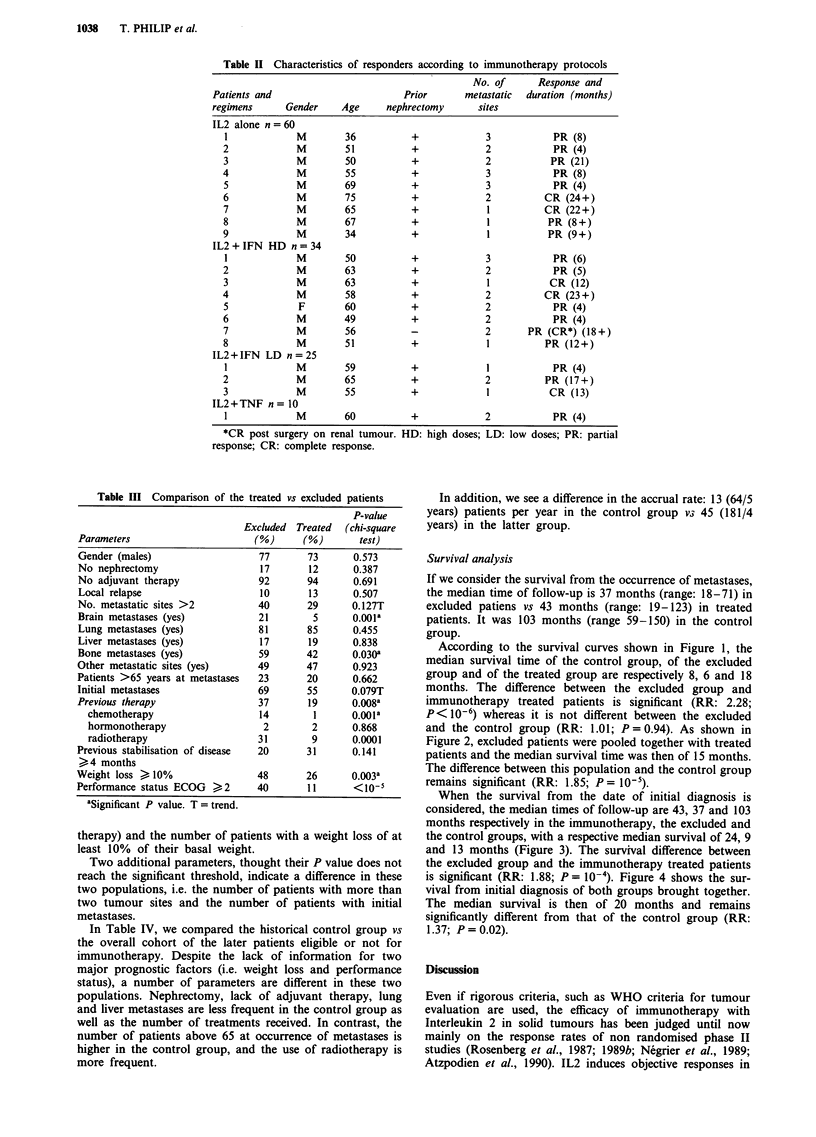
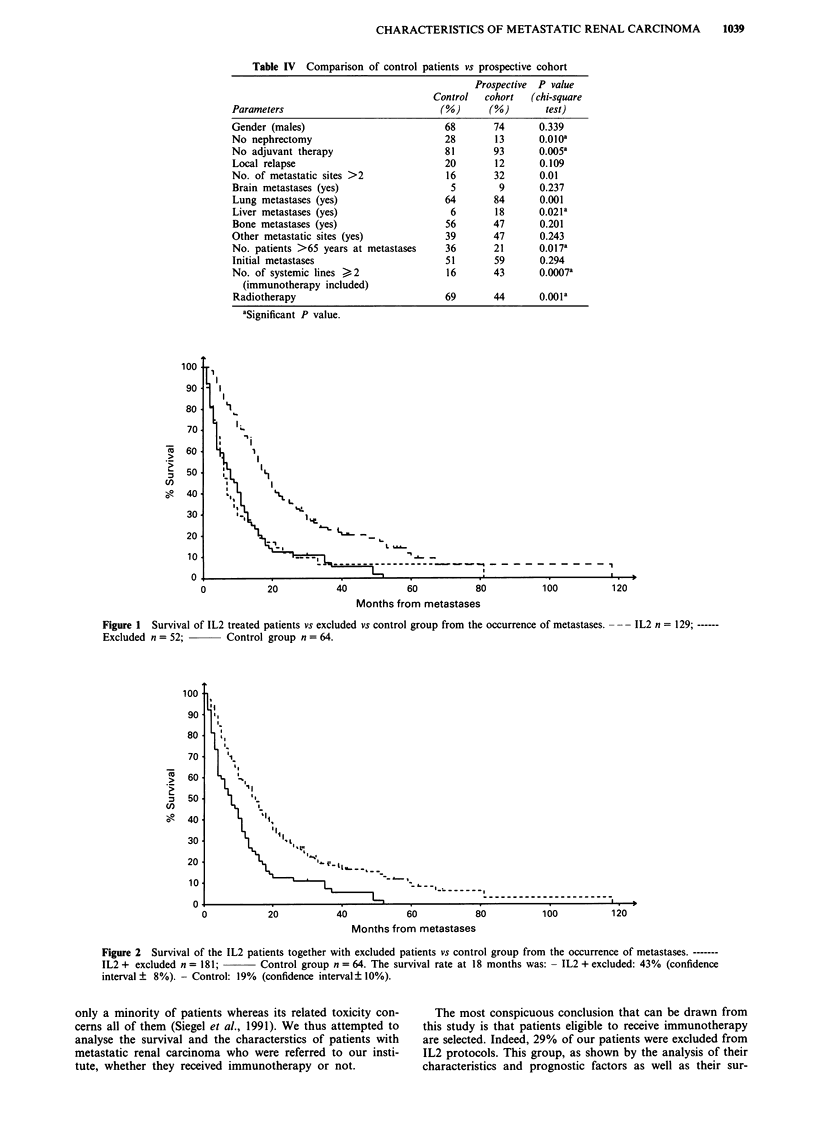
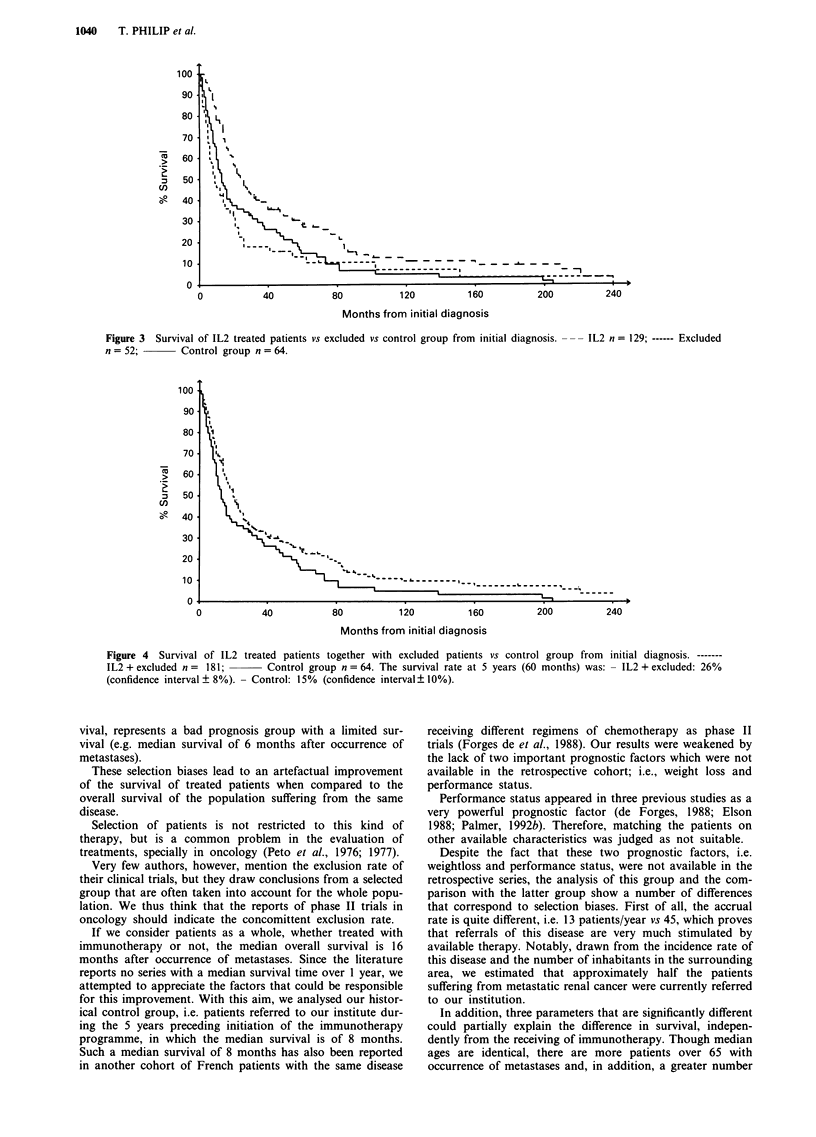
This study was performed with the aim of discovering the characteristics and survival of patients with metastatic renal carcinoma who undergo immunotherapy with an Interleukin 2 based regimen. One hundred and eighty-one patients with metastatic renal carcinoma were referred to our institute from October 1987 until August 1991; 129 were treated with Interleukin 2 with or without Interferon alpha in three successive protocols. Fifty-two patients were not treated with immunotherapy due to the exclusion criteria of the protocols. Sixty-four patients with the same disease who had been referred to our institute before the initiation of this programme (1982, 1987) were also analysed as a control group. The main characteristics of the three different cohorts of patients were analysed and compared with univariate statistical tests; the median survival of the patients was calculated and compared. The referral rate increased from 13 a year to 45 a year while the IL2 trials were being conducted. Patients treated with cytokines have a median survival of 18 months after occurrence of metastases, compared to 6 and 8 months, respectively, in excluded patients and the control group. This parameter is of 15 months when the 181 patients, treated with cytokines or not, are considered. The survival of treated vs excluded patients is significantly different (P < 10(-6); so is the survival of the 181 patients recently included when compared to the historical group (P:10(-5). When the 181 recent patients are compared to the historical control group, a number of differences appear in their characteristics, which prevent us from drawing any conclusion about the role of immunotherapy in the improvement of survival observed. This study clearly evidences the selection of the patients receiving immunotherapy and the modification in referrals of a disease induced by a new available therapy. This emphasises the need for prospective studies in this setting.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atzpodien J., Körfer A., Franks C. R., Poliwoda H., Kirchner H. Home therapy with recombinant interleukin-2 and interferon-alpha 2b in advanced human malignancies. Lancet. 1990 Jun 23;335(8704):1509–1512. doi: 10.1016/0140-6736(90)93039-r. [DOI] [PubMed] [Google Scholar]
- Bergerat J. P., Herbrecht R., Dufour P., Jacqmin D., Bollack C., Prevot G., Bailly G., de Garis S., Juraschek F., Oberling F. Combination of recombinant interferon alpha-2a and vinblastine in advanced renal cell cancer. Cancer. 1988 Dec 1;62(11):2320–2324. doi: 10.1002/1097-0142(19881201)62:11<2320::aid-cncr2820621111>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Droz J. P., Theodore C., Ghosn M., Lupera H., Piot G., De Forges A., Klink M., Rouesse J., Amiel J. L. Twelve-year experience with chemotherapy in adult metastatic renal cell carcinoma at the Institut Gustave-Roussy. Semin Surg Oncol. 1988;4(2):97–99. [PubMed] [Google Scholar]
- Elson P. J., Witte R. S., Trump D. L. Prognostic factors for survival in patients with recurrent or metastatic renal cell carcinoma. Cancer Res. 1988 Dec 15;48(24 Pt 1):7310–7313. [PubMed] [Google Scholar]
- Krown S. E. Interferon treatment of renal cell carcinoma. Current status and future prospects. Cancer. 1987 Feb 1;59(3 Suppl):647–651. doi: 10.1002/1097-0142(19870201)59:3+<647::aid-cncr2820591313>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Maldazys J. D., deKernion J. B. Prognostic factors in metastatic renal carcinoma. J Urol. 1986 Aug;136(2):376–379. doi: 10.1016/s0022-5347(17)44873-7. [DOI] [PubMed] [Google Scholar]
- Moertel C. G. On lymphokines, cytokines, and breakthroughs. JAMA. 1986 Dec 12;256(22):3141–3141. [PubMed] [Google Scholar]
- Negrier M. S., Pourreau C. N., Palmer P. A., Ranchere J. Y., Mercatello A., Viens P., Blaise D., Jasmin C., Misset J. L., Franks C. R. Phase I trial of recombinant interleukin-2 followed by recombinant tumor necrosis factor in patients with metastatic cancer. J Immunother (1991) 1992 Feb;11(2):93–102. doi: 10.1097/00002371-199202000-00003. [DOI] [PubMed] [Google Scholar]
- Negrier S., Philip T., Stoter G., Fossa S. D., Janssen S., Iacone A., Cleton F. S., Eremin O., Israel L., Jasmin C. Interleukin-2 with or without LAK cells in metastatic renal cell carcinoma: a report of a European multicentre study. Eur J Cancer Clin Oncol. 1989;25 (Suppl 3):S21–S28. [PubMed] [Google Scholar]
- Négrier S., Mercatello A., Bret M., Thiesse P., Blay J. Y., Coronel B., Merrouche Y., Oskam R., Franks C. R., Clavel M. Intravenous interleukin-2 in patients over 65 with metastatic renal carcinoma. Br J Cancer. 1992 May;65(5):723–726. doi: 10.1038/bjc.1992.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osband M. E., Ross S. Problems in the investigational study and clinical use of cancer immunotherapy. Immunol Today. 1990 Jun;11(6):193–195. doi: 10.1016/0167-5699(90)90080-s. [DOI] [PubMed] [Google Scholar]
- Palmer P. A., Vinke J., Evers P., Pourreau C., Oskam R., Roest G., Vlems F., Becker L., Loriaux E., Franks C. R. Continuous infusion of recombinant interleukin-2 with or without autologous lymphokine activated killer cells for the treatment of advanced renal cell carcinoma. Eur J Cancer. 1992;28A(6-7):1038–1044. doi: 10.1016/0959-8049(92)90450-g. [DOI] [PubMed] [Google Scholar]
- Palmer P. A., Vinke J., Philip T., Negrier S., Atzpodien J., Kirchner H., Oskam R., Franks C. R. Prognostic factors for survival in patients with advanced renal cell carcinoma treated with recombinant interleukin-2. Ann Oncol. 1992 Jun;3(6):475–480. doi: 10.1093/oxfordjournals.annonc.a058239. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976 Dec;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip T., Mercatello A., Negrier S., Philip I., Rebattu P., Kaemmerlin P., Gaspard M., Tognier E., Combaret V., Bijmann J. T. Interleukin-2 with and without LAK cells in metastatic renal cell carcinoma: the Lyon first-year experience in 20 patients. Cancer Treat Rev. 1989 Jun;16 (Suppl A):91–104. doi: 10.1016/0305-7372(89)90028-5. [DOI] [PubMed] [Google Scholar]
- Quesada J. R., Swanson D. A., Trindade A., Gutterman J. U. Renal cell carcinoma: antitumor effects of leukocyte interferon. Cancer Res. 1983 Feb;43(2):940–947. [PubMed] [Google Scholar]
- Ritchie A. W., deKernion J. B. The natural history and clinical features of renal carcinoma. Semin Nephrol. 1987 Jun;7(2):131–139. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Aebersold P. M., Linehan W. M., Seipp C. A., White D. E. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. P., Puri R. K. Interleukin-2 toxicity. J Clin Oncol. 1991 Apr;9(4):694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- West W. H., Tauer K. W., Yannelli J. R., Marshall G. D., Orr D. W., Thurman G. B., Oldham R. K. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987 Apr 9;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- Yagoda A. Chemotherapy of renal cell carcinoma: 1983-1989. Semin Urol. 1989 Nov;7(4):199–206. [PubMed] [Google Scholar]
- de Forges A., Rey A., Klink M., Ghosn M., Kramar A., Droz J. P. Prognostic factors of adult metastatic renal carcinoma: a multivariate analysis. Semin Surg Oncol. 1988;4(3):149–154. doi: 10.1002/ssu.2980040302. [DOI] [PubMed] [Google Scholar]


