Abstract
G1 is a crucial phase of cell growth because the decision to begin another mitotic cycle is made during this period. Occurrence of DNA damage in G1 poses a particular challenge, because replication of damaged DNA can be deleterious and because no sister chromatid is present to provide a template for recombinational repair. We therefore have studied the response of Schizosaccharomyces pombe cells to UV irradiation in early G1 phase. We find that irradiation results in delayed progression through G1, as manifested most critically in the delayed formation of the pre-replication complex. This delay does not have the molecular hallmarks of known checkpoint responses: it is independent of the checkpoint proteins Rad3, Cds1, and Chk1 and does not elicit inhibitory phosphorylation of Cdc2. Irradiated cells eventually progress into S phase and arrest in early S by a rad3- and cds1-dependent mechanism, most likely the intra-S checkpoint. Caffeine alleviates both the intra-G1- and intra-S-phase delays. We suggest that intra-G1 delay may be widely conserved and discuss significance and possible mechanisms.
Keywords: UV radiation, DNA replication, G1 phase, synchronization, Cdc10
When supplied with appropriate nutrients, all growing cells, from simple prokaryote to complex eukaryote, go through an obligatory cell cycle consisting of alternating periods of DNA replication (S phase), segregation of the chromosomes and nuclear division (mitosis), and cell division. The order of events is crucial and is monitored by molecular mechanisms termed checkpoints. A checkpoint inhibits or delays an event of the cell cycle if an upstream event has not been completed properly or if the DNA is damaged (1).
Several different DNA damage and DNA replication checkpoints have been identified and characterized in the fission yeast Schizosaccharomyces pombe (2, 3). They include mechanisms to inhibit mitosis when the DNA is damaged (the G2/M checkpoint) or when S phase has not been completed (the S/M checkpoint), as well as a mechanism to inhibit ongoing DNA replication when the DNA is damaged (the intra-S checkpoint). These three checkpoints have several molecular determinants in common. First, all of them depend on a set of genes called the checkpoint rad genes. Second, they all are mediated by one or both of two protein kinases, Chk1 and Cds1. Third, a downstream target for all of them is the Cdc2 kinase, which is kept phosphorylated on tyrosine-15 and, thus, is inactivated when the checkpoints are induced.
The Cds1 and Chk1 protein kinases are central players in all of the DNA damage and replication checkpoints characterized in S. pombe, but they have different functions in the different checkpoints. The S/M and intra-S checkpoints both depend on Cds1, which is activated only in S phase (4, 5). The Cds1 protein is phosphorylated by a process dependent on Rad3 and Mrc1 (6, 7). Chk1 is phosphorylated after DNA damage in late S or in G2 phase in a process dependent on Rad3 and Crb2 (8–10), and this phosphorylation has been taken as a diagnostic sign of checkpoint activation. In human cells, the Rad3 homolog ATR binds and phosphorylates CHK1 (11), and the ATM and ATR proteins phosphorylate the Cds1 homolog CHK2 (12), emphasizing the conservation of checkpoint mechanisms through evolution.
Despite the wealth of information regarding cellular checkpoints in general and DNA damage checkpoints in particular, the nature of such mechanisms operating during G1 remains poorly understood. The effects of DNA damage during the G1 phase are particularly interesting for several reasons. First, the cell decides whether to proceed into another cycle during G1. Second, entry into S phase with unrepaired damage will lead to mutations and genetic instability, two hallmarks of cancer development. Third, there is no sister chromatid (and, in a haploid organism such as S. pombe, no homolog) to serve as a template for recombinational repair.
In several organisms, introduction of DNA damage during G1 leads to delayed start of bulk DNA replication, as seen in budding yeast (13), Xenopus (14), and human cells (reviewed in refs. 15 and 16). The molecular mechanisms of such checkpoints and, in particular, whether arrest occurs within G1, at the G1/S boundary, or early in S phase are not defined. There are no reports of a checkpoint arresting S. pombe cells in G1 after DNA damage. One study (17) did observe a delay in S phase after infliction of DNA damage in G1; this phenomenon was not fully characterized but was proposed to result from the intra-S checkpoint (17). Thus, the detailed nature of cellular responses to DNA damage in G1 remains to be determined. In the present work we have used several complementary methods to study progression through G1 and into S phase in UV-irradiated S. pombe cells.
Materials and Methods
Yeast Strains. All strains are derivatives of the L972 strain. Checkpoint mutant strains containing insertions of ura4+ in the relevant genes were received from T. Carr (University of Sussex, Brighton, U. K.). The cdc21::GFP construct was from S. Kearsey (Oxford University, Oxford) (18). The strains were constructed by standard genetic methods.
Cell Growth. All basic growth and media conditions were as described (19). Mutant cdc10-M17 cells were grown in standard Edinburgh minimal medium 2 (EMM2) at 25°C, synchronized by incubation for 4 h at 36°C, shifted to 25°C, and immediately UV-irradiated. When noted, caffeine was present at 10 mM.
Flow Cytometry. About 107 cells were spun down and fixed in 70% ethanol. Cells were processed for flow cytometry as described (20) and stained with SYTOX Green (http://pingu.salk.edu/users/forsburg/lab.html). Flow cytometry was performed with a Becton Dickinson FACSCalibur with 488-nm excitation light.
UV Irradiation. Cells suspended in a thin layer (3 mm) of rapidly stirred liquid medium were irradiated at 20–25°C with 254-nm UV light. The dose was measured with a radiometer (Ultraviolet Products, San Gabriel, CA), and an exposure time of 4 min gave an incident dose of ≈1,000 J/m2. The incident dose does not reflect the dose absorbed by the cells because UV light of this wavelength penetrates poorly into water. However, because irradiation conditions were constant, the incident dose was proportional to the absorbed dose.
Cds1 Kinase Assays. Immunoprecipitates of Cds1 were used to measure Cds1 kinase activity, with myelin basic protein (MBP) as substrate, as described elsewhere (5). As a control for equal amounts of Cds1 in the kinase assay, a fraction of each sample was subjected to immunoblotting with Cds1 antibodies.
2D Gel Analysis. Purified genomic DNA was digested with HindIII and KpnI and analyzed as described before (21). Equal amounts of DNA were loaded in each lane, and loading was checked after staining the 1D and 2D gels with ethidium bromide. All samples from one experiment were blotted onto the same membrane, which was probed with the 3-kb HindIII–KpnI fragment from the rRNA-encoding DNA repeats of S. pombe (22).
RNA Analysis. Total RNA was isolated, run in an agarose gel in formaldehyde, and blotted onto a nitrocellulose membrane (NitroPure, Osmonics, Westborough, MA). A PCR product of the entire cig2 ORF was inserted into a pGEM-3 multicloning site (Promega), and the resulting plasmid was used to obtain a 32P-labeled single-stranded probe by using T7 RNA polymerase. The probe was hybridized to the membrane, which was washed and exposed to a PhosphorImager screen.
Analysis of Pre-Replication Complex (RC) Formation. The cell wall was partly removed with Zymolyase, the cells were permeabilized, extracted with Triton X-100, and fixed, and the presence and location of remaining Mcm4-GFP were determined by fluorescence microscopy, essentially as described (18).
Results
UV Irradiation in G1. We have used flow cytometry to show, in three different growth situations, that exposure to UV light in G1 delays a subsequent start of bulk DNA replication.
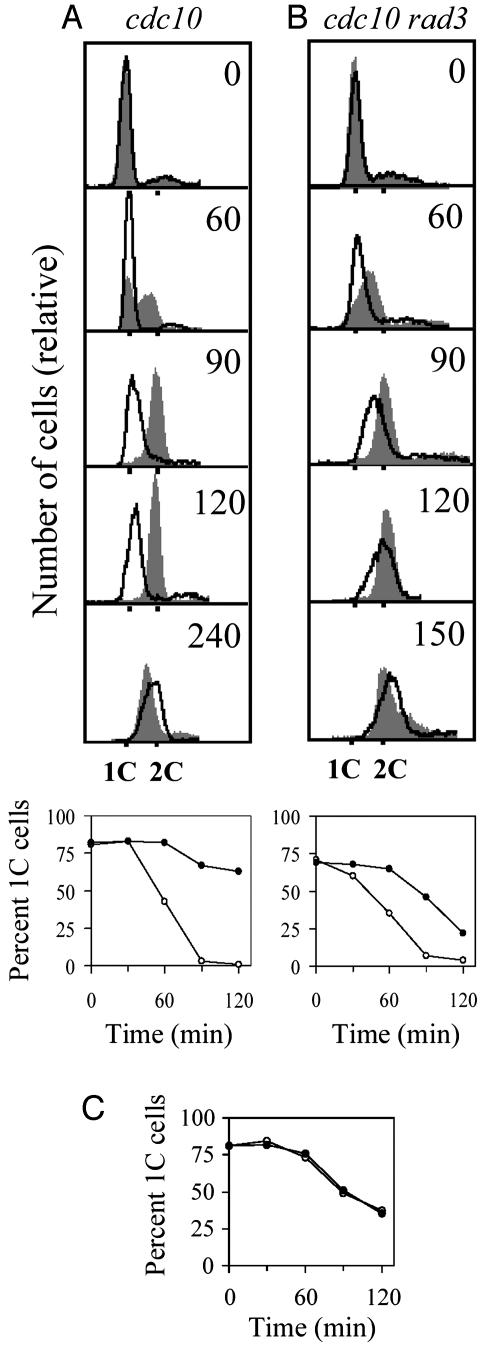
Cells synchronized in G1. Temperature-sensitive (ts) cdc10-M17 cells were synchronized in early G1 by a 4-h shift to 36°C, released at 25°C, and irradiated immediately. Cdc10 induces transcription of a set of genes required for progression through G1 into S phase (23–25). These genes, including cdc18, cdt1, and cig2, regulate the preparation for DNA replication, most importantly the formation of the pre-RC (18, 26). The absence of Cdc10 function prevents formation of the pre-RC and therefore arrests the cells before initiation of DNA replication (18, 27, 28). In our experiments, almost all of the cdc10ts cells had a haploid (1C) DNA content after 4 h of incubation at 36°C (Fig. 1A Upper). These cells were shifted to 25°C and exposed to a dose of UV light (1,100 J/m2) that gave a cell survival rate of ≈15% (data not shown), and the DNA content of individual cells was monitored by flow cytometry during the subsequent incubation at 25°C. In the DNA histograms from the unirradiated cells, an increase from 1C to 2C in DNA content occurred ≈60 min after the temperature downshift. UV irradiation delayed the transition by >90 min; only at 240 min had all of the irradiated cells finished S phase (Fig. 1A).
Fig. 1.
Demonstration of a UV-inducible delay is shown. (A and B Upper) Synchronized cdc10-M17 (A) and cdc10-M17 rad3 (B) cells were UV-irradiated, and samples were removed for flow cytometry at the times indicated (in min). DNA histograms are shown for unirradiated control cells (filled profile) and irradiated cells (open profile with bold outline). (A and B Lower) Quantification of the fraction of cells with a 1C complement of DNA in the respective DNA histograms. ○, Control cells; •, irradiated cells. (C) Quantification of histograms from cdc10 cells irradiated and incubated in the presence of caffeine.
Cells synchronized at G2/M. To exclude the possibility that the cdc10 mutation or the synchronization method used unduly affected our experiments, we investigated ts cdc25-22 cells synchronized at the G2/M border. The cells were released at the permissive temperature and irradiated as they passed into G1. The synchronized cdc25ts cells displayed a radiation-induced delay with 1C DNA that lasted for >1 h (Supporting Text and Fig. 5, which are published as supporting information on the PNAS web site, www.pnas.org).
Cycling cells. To further exclude the possibility that the response described did not result from artifacts induced by the use of cell-cycle mutants, we studied cycling, unperturbed, wild-type cells. Cells irradiated in late G1 phase did not delay with a 1C DNA content but were arrested inside S phase, as determined by flow cytometry (Supporting Text and Fig. 6, which are published as supporting information on the PNAS web site). In contrast, cells irradiated in early G1 delayed by ≈60 min before performing bulk DNA replication.
The UV-Irradiated Cells Are Delayed in G1 Phase. The flow cytometry analyses described above cannot discriminate between cells in G1 with a true 1C DNA content and early S-phase cells that have replicated a small fraction of their DNA. Therefore, we performed 2D gel electrophoresis of DNA isolated from cdc10ts cells treated as above. We investigated the status of the early replication origin ars3001, which is located in the rRNA-encoding DNA repeats. The experiment was repeated several times in separate experiments, and the results were as follows. In the unirradiated control cells, feeble but detectable replication activity was observed at the time of temperature downshift, probably reflecting the fact that a small fraction of the cells had leaked past the cdc10 arrest point. A strong replication activity always was observed 60 min after release (Fig. 2A), in agreement with the shift in DNA content from 1C to 2C observed by flow cytometry (Fig. 1). The ars3001 origin was replicated passively, as evidenced by a Y-arc (arrow, Fig. 2A), and served as an origin, as evidenced by the bubble-arc (arrowhead, Fig. 2A). In irradiated cells, there was a low and increasing replication activity after 30, 60, and 90 min, but the activity was much weaker than that found in unirradiated cells. These results provide strong evidence that UV irradiation induces a transient arrest before the start of DNA replication.
Fig. 2.
Arrested cells have not replicated the early origin ars3001. (A) Mutant cdc10ts cells were incubated at 36°C for 4 h before release at 25°C. Samples were taken before release and at the times indicated for control (Left) and UV-irradiated (Right) cells. DNA was purified from each sample and subjected to 2D electrophoresis, blotting, and probing with an ars3001-specific probe. All panels are from the same experiment and the same blot. (B) Synchronized cdc10ts mutant cells carrying the Mcm4-GFP allele were treated as described in Fig. 1, and samples were removed at the time of irradiation and after1hof incubation at 25°C. The cells were permeabilized, and unbound Mcm4 was extracted with detergent. GFP fluorescence was detected, and the cells were classified as either negative (Left) or positive (Right). (C) Percentage of positive cells, after 0 min and after 60 min, with or without UV. Standard errors are indicated.
Mcm4 Does Not Bind to Chromatin During G1 Arrest. To further investigate the UV-induced G1 arrest point, we examined UV-treated cells with respect to the status of the Mcm proteins, which form the pre-RC by loading onto future replication origins in a step-dependent manner on a functional origin recognition complex, on cdc10-dependent transcription and on Cdc18 (18). To this end, we used a cdc10-M17 strain in which the Mcm4 protein is labeled with GFP. The presence of chromatin-bound Mcm4 was detected by fluorescence microscopy after extraction of unbound Mcm4 in permeabilized cells. In unirradiated cells, very few cells were positive for GFP after4hofincubation at 36°C (0 h; Fig. 2B); then, by 60 min after release at 25°C, a time at which most cells had begun DNA replication (Fig. 1 and Fig. 2A), ≈80% contained chromatin-bound Mcm4-GFP. In contrast, in cells UV-irradiated and released at 25°C, only a small fraction (14%) contained significant levels of Mcm4-GFP staining at 60 min. These cells likely correspond to the minority of cells that display replication activity of the early origins at this time point (UV60, Fig. 2A). We conclude that UV irradiation in the cdc10 block point produces a transient arrest in early G1, before loading of the Mcm proteins, and that this transient arrest results in delayed S-phase entry.
Rad3 Is Required for a Portion of the UV-Induced Delay in Bulk DNA Replication. Many DNA damage responses are mediated by homologs of the phosphatidylinositol 3-kinase-like (PIK) kinases ATR and ATM. To assess the role of the ATR homolog Rad3 in UV-induced G1 arrest, a cdc10-M17 rad3 double mutant was analyzed by flow cytometry. In this strain, the delay in leaving the 1C population was reduced relative to that found in the isogenic rad3+ strain, but, reproducibly, a 40-min delay still was observed (Fig. 1B). Thus, approximately half of the UV-induced delay in progression into full S phase is rad3-dependent, whereas the other half is rad3-independent. This result is corroborated by results from cdc25ts cells synchronized in G2/M and irradiated in early G1: the delay in increase in bulk DNA synthesis was not removed by a rad3 mutation (Fig. 5).
Cds1, but Not Chk1, Is Required for a Portion of the UV-Induced Delay in Bulk DNA Replication. The known DNA damage checkpoint responses in S. pombe are mediated by the Cds1 or Chk1 kinases (see above). We therefore similarly assessed the involvement of these proteins in the response to UV irradiation in synchronized cdc10ts or cdc25ts cells. Mutant cdc10ts chk1 cells exhibited the same delay on entry into S phase as isogenic chk1+ cells, as defined by flow cytometry (Fig. 3A), whereas in cdc10ts cds1 cells the delay was reduced from >90 min to ≈50 min (Fig. 3B). Triple-mutant cds1 chk1 cdc10ts cells behaved like cds1 cdc10ts cells (Fig. 3C). Analogous results were obtained in cdc25ts cells (data not shown). These results demonstrate that Chk1 is not involved in the response to UV irradiation in G1, whereas Cds1 seems to have some role. Interestingly, cds1 and rad3 mutations confer very similar defects.
Fig. 3.
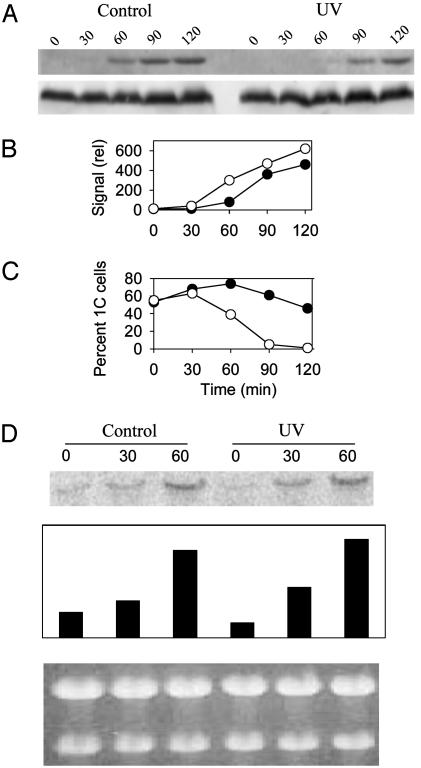
Cds1, but not Chk1, is involved in the delay. (A–C) Synchronized cdc10ts cells carrying chk1 (A), cds1 (B), or cds1 chk1 (C) mutations were treated as described in Fig. 1. Quantification of the fraction of 1C cells is shown, as described in the legend to Fig. 1. (D) Synchronized cdc10ts mutant cells were UV-irradiated, and samples were taken after 0, 60, and 90 min and analyzed for Cds1 kinase activity. (Upper) The relative amounts of Cds1 protein used in the kinase assay were estimated by immunoblotting. (Lower) The kinase activity was measured by phosphorylation of myelin basic protein (MBP). The hydroxyurea (HU)-treated cells gave such a strong kinase signal that we reduced the intensity of the exposure of this lane to visualize the MBP bands in all lanes. Quantification showed that the signal from HU-treated cells was 8.5-fold higher than that from the UV90 sample.
UV Irradiation Results in Activation of Cds1 Kinase, but only at the Onset of S Phase. We also investigated activation of Cds1 in irradiated cdc10ts cells by assaying the level of Cds1 kinase activity. In unirradiated control cells, no activity could be detected for the first 60 min after release from arrest; a low but detectable level of kinase activity then was observed at 60 and 90 min after release (Fig. 3D), at which times the majority of cells have entered bulk S phase as defined by flow cytometry (Fig. 1). This low level may reflect a slight activation of the Cds1 kinase even during a normal S phase. In irradiated cells, strong Cds1 activation could be detected, but only after 90 min. The level of Cds1 kinase activity observed after UV-irradiation was ≈10-fold weaker than that observed after treatment of asynchronous cells with hydroxyurea (HU) for 3 h (Fig. 3D). These data demonstrate that Cds1 is activated after UV irradiation, but after the time when unirradiated cells have entered S phase. Significantly, Cds1 remains unphosphorylated during the initial 60 min after UV irradiation and, thus, throughout the period when formation of the pre-RC is inhibited (see above). In irradiated cells, the time of phosphorylation corresponds to the period when a low level of replication activity begins to be detected (Fig. 2 A) and is before the onset of bulk DNA replication as defined by the exit of cells from 1C DNA content (Fig. 1). Therefore, the timing and level of Cds1 activation most likely reflect that more and more irradiated cells start DNA replication after 60 min, thus activating the intra-S checkpoint and Cds1 activity, which fits with the cds1 mutant analysis (see above).
We could detect no activation of Chk1 (data not shown), which was measured as a mobility shift in immunoblots (9), in accordance both with our finding that a chk1 deletion does not affect the checkpoint (see above) and with a previous report that Chk1 is not activated by UV irradiation in G1 (9).
UV Irradiation Delays Cdc2 Phosphorylation. Cdc2 kinase is required for entry into S phase (29) and consequently for the firing of early replication origins. During an unperturbed cell cycle, Cdc2 phosphorylation occurs shortly after Cdc2 activity has brought the cells into S phase (30). The purpose of this phosphorylation is to keep the Cdc2 kinase activity low to prevent the too early execution of mitosis (31). Analogously, in cells arrested in the intra-S, the S/M, or the G2/M checkpoints, mitosis is inhibited by Cdc2 phosphorylation (32–34).
We have measured directly the timing of Cdc2 protein phosphorylation after exposure to UV in the Cdc10 block point. Phosphorylation of Cdc2 could be detected in extracts of unirradiated cells ≈60 min after release from the block (Fig. 4 A and B), i.e., as they enter S phase (Fig. 4C), in agreement with previous findings (30). In contrast, irradiated cells consistently exhibited phosphorylated Cdc2 ≈30 min later, ≈90 min after release from the block (Fig. 4 A and B). The timing of Cdc2 phosphorylation is essentially the same as the timing of Cds1 activation (Fig. 3D) and activation of early origins (Fig. 2 A).
Fig. 4.
The Cdc2 protein is not phosphorylated in the initial phases of the delay. Synchronized cdc10ts mutant cells were UV-irradiated, and samples were taken for flow cytometry and for immunoblotting at different time points after irradiation. (A) The immunoblots were probed with antibodies to phosphorylated Cdc2 (Upper) and to total Cdc2 protein (Lower). (B) Quantification of the immunoblot, with correction for loading and using total Cdc2 as the loading control. (C) Percentage of 1C cells, as determined by flow cytometry. ○, Unirradiated control cells; •, irradiated cells. (D) A blot of total RNA probed with a cig2-specific probe (Top). Quantification is shown (Middle), as is the gel after ethidium bromide staining (Bottom), to demonstrate even loading.
Taken together, these results imply that the phosphorylation status of Cds1 and Cdc2 remains unaltered during the initial 60 min after UV irradiation and, thus, throughout the period when MCM loading is inhibited (Fig. 2B). Then, after this delay, Cds1 and Cdc2 become phosphorylated, concomitant with a low level of replication activity. Finally, after another 60–90 min, bulk DNA replication occurs.
G1-Specific Transcription Is Not Affected. The G1/S checkpoint in Saccharomyces cerevisiae works by inhibiting the SWI6-dependent transcription required for S-phase entry, which leads to down-regulation of G1 cyclins and delayed entry into S phase (35). In S. pombe, entry into S phase requires Cdc10-dependent transcription. We therefore investigated whether UV irradiation affects expression of cig2 or cdc18, two genes whose transcription requires Cdc10. The Cdc10ts protein becomes active at downshift to 25°C, and Cdc10-dependent transcription occurs shortly thereafter in unirradiated cells, allowing preparation for S phase. Total RNA was purified from cells arrested in G1, released, and irradiated as described above. The RNA was run out in an agarose gel, blotted, and hybridized to a cig2-specific probe, and the blot shows that cig2 transcription was unaffected by UV irradiation (Fig. 4D). Similar data were obtained for the transcription of cdc18 (data not shown).
Caffeine Abolishes All UV-Induced G1 Progression Defects. Caffeine has a wide variety of effects on cellular metabolism and is known to eliminate diverse cell-cycle-mediated checkpoint responses (36–38). We therefore determined the effects of caffeine on the delays induced by UV-irradiation in G1. We find that caffeine totally removed the difference in the timing of the onset of bulk DNA replication, as seen by flow cytometry. In the case of synchronized cdc10ts cells, the DNA histograms from irradiated and unirradiated cells were almost identical, implying that the delay was totally abolished (Fig. 1C). Similarly, irradiated and nonirradiated cycling cells entered S phase at the same time in the presence of caffeine (Fig. 6E). The last part of the delay observed in the absence of caffeine, ascribed to the intra-S checkpoint, is most likely abolished because of caffeine action on Rad3 activity (38). Because caffeine is known to disturb many cellular reactions, there are several possible explanations for its effect on G1 progression, but its effect here provides an indication of an interesting underlying process.
γ Irradiation in G1 Does Not Affect S-Phase Entry. To determine the effect of ionizing radiation in G1 phase, cdc10ts cells were synchronized as above and exposed to x-irradiation (cell survival rate of ≈20%), and the kinetics of bulk DNA replication was followed by flow cytometry. No delay in entry into S phase could be detected, but the cells were delayed inside S phase (data not shown). We conclude that the mechanism to delay cells in G1 is not sensitive to ionizing radiation.
Discussion
We have UV-irradiated fission yeast cells in G1 and investigated the consequences for cell-cycle progression. The first detected response is a delay in MCM loading, which implies that UV irradiation impedes progression through G1. Bulk DNA replication also is delayed, but for a much longer time, reflecting the presence of a second, later progression defect. During the intervening period, a low level of replication activity can be observed. These results point to a two-phase response. First, there is a delay in progression from early to late G1. S phase then begins, as signaled by a low level of replication activation, but is blocked for an extended period. After this second block, bulk DNA replication occurs.
This interpretation is confirmed and extended by genetic data and studies of Cds1 and Cdc2 phosphorylation. Neither protein is phosphorylated during the period when Mcm4 loading is blocked. Thus, these proteins are not involved in the first phase of the delay response. Both proteins then are phosphorylated during the period of low replication activity. Both are already known to be phosphorylated in the intra-S checkpoint, which is also characterized by a low residual level of initial DNA replication. Thus, the second phase of the response to UV irradiation likely corresponds to a standard intra-S DNA damage response. Accordingly, Cds1 and Rad3, both of which are implicated in the intra-S checkpoint, are required for a portion of the delay in onset of bulk S phase, as expected if the corresponding mutations specifically eliminate the second phase of the response.
This is, to our knowledge, the first demonstration that UV-irradiation in early G1 produces a delay in progression through that stage, before the onset of DNA replication. The loading of Mcm proteins onto chromatin, which we interpret as formation of the pre-RC, is affected, showing that the response is affecting progression from early to mid-G1.
Failure to detect such a delay in previous studies of S. pombe is probably due to the fact that the G1 phase is extremely short in the rich media commonly used for S. pombe growth. In the current study, we have used cells arrested in G1 phase as well as cells grown on a less accessible nitrogen source, where they have a longer G1 phase (Supporting Text and ref. 39). Slow growth may mimic the conditions frequently encountered in nature, where S. pombe cells could make good use of a G1 response to genotoxic assaults like UV irradiation.
Most previous studies of the effects of DNA damage in G1 in other organisms rely on measurements of bulk DNA replication, of cellular DNA content by flow cytometry, or, in budding yeast, of sensitivity to pheromone or morphological criteria such as budding. However, none of these methods can directly identify the step where arrest occurs or, specifically, whether DNA replication has started. Thus, it remains to be determined whether these responses reflect activation of the intra-S checkpoint or whether an intra-G1 delay is (also) involved. In particular, it is not clear whether budding yeast cells arrest in G1 phase after UV irradiation in G1 (40, 41).
There are some reports suggesting that other eukaryotic cells have similar delay mechanisms to that described here: a nonclassical delay in G1 has been suggested for mammalian cells (42), in which origin firing and S-phase entry were inhibited by a previously uncharacterized mechanism. Also, the classical G1 checkpoint in mammalian cells depends on p53, but in its absence there is still a significant delay in S phase (43). Furthermore, data from budding yeast give evidence of a noncheckpoint G1 arrest (13, 44–46).
The nature of the G1 progression delay observed in the current work is intriguing, with respect to both its target and its mechanism. The relevant target of the UV-induced response may be DNA, but other targets (e.g., proteins, RNA, membrane) and other functions (transport, translation) are not excluded. In any case, because γ irradiation does not produce an analogous delay, the response does not involve loss of covalent DNA integrity. Because none of the known classical checkpoint proteins are implicated as mediators or targets of the delay, it is likely that it does not comprise a classical DNA damage checkpoint response. The G1 delay is removed by caffeine, which is known to inhibit some checkpoint responses and phosphatidyl-inositol 3-kinase-like (PIK) kinases (38). The delay described here does not involve the PIK kinase Rad3 (above) and Tel1 (data not shown). Recent data suggest that checkpoint inhibition by caffeine does not operate through PIK inhibition (47).
The existence of a progression response in G1 phase is of special general importance because progression through this period includes Start, which seems to define the onset of the mitotic cell cycle, and because alterations in progression through this stage lie at the heart of cell-cycle deregulation and cancer. The unusual nature of the UV-induced progression delay observed in this work has prompted us to investigate other reports of unusual G1 progression phenomena. Among these, two are particularly striking. First, there are several reports that G1/S progression is regulated, positively or negatively, by chromosomal proteins that originally were identified by their roles in meiotic or mitotic sister chromatid cohesion or meiotic interhomolog interactions, none of which can take place in mitotic G1 cells (48–54). The human homologs of these proteins, the chromosome morphogenesis protein Spo76/BIMD/AS3 and the securin Pds1/Cut2, are both implicated as tumor suppressor proteins, and modulation of progression through G1 by these proteins can be directly linked to cancer (51, 52). Second, in budding yeast it has been reported that a cyclic transcription pattern can occur in cells that appear to lack cyclins (55), and this process has been related to cyclic gene-expression patterns in other noncycling cells (56). Thus, our finding of a checkpoint-independent G1 progression control is not unprecedented and could be related to other G1 progression processes that are of fundamental importance for several aspects of cellular life.
Supplementary Material
Acknowledgments
We thank J. A. Sandvik and M. O. Haugli for technical assistance, J. Sogo and his lab members for hospitality and instructions in 2D gel analysis, I. Hagan and O. Nielsen for help with the synchronization techniques, J. Huberman for communicating unpublished information, H. Lindsay for the Cds1 antiserum, and T. Carr and S. Kearsey for supplying strains. This work was supported by The Norwegian Research Council, The Norwegian Cancer Society, and the Odd Fellows Society.
Abbreviations: RC, replication complex; ts, temperature-sensitive.
References
- 1.Hartwell, L. H. & Weinert, T. A. (1989) Science 246, 629–634. [DOI] [PubMed] [Google Scholar]
- 2.Caspari, T. & Carr, A. M. (1999) Biochimie 81, 173–181. [DOI] [PubMed] [Google Scholar]
- 3.Huberman, J. A. (1999) Prog. Nucleic Acid Res. Mol. Biol. 62, 369–395. [DOI] [PubMed] [Google Scholar]
- 4.Murakami, H. & Okayama, H. (1995) Nature 374, 817–819. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay, H. D., Griffiths, D. J., Edwards, R. J., Christensen, P. U., Murray, J. M., Osman, F., Walworth, N. & Carr, A. M. (1998) Genes Dev. 12, 382–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcasabas, A. A., Osborn, A. J., Bachant, J., Hu, F., Werler, P. J., Bousset, K., Furuya, K., Diffley, J. F., Carr, A. M. & Elledge, S. J. (2001) Nat. Cell Biol. 3, 958–965. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka, K. & Russell, P. (2001) Nat. Cell Biol. 3, 966–972. [DOI] [PubMed] [Google Scholar]
- 8.Saka, Y., Esashi, F., Matsusaka, T., Mochida, S. & Yanagida, M. (1997) Genes Dev. 11, 3387–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinho, R. G., Lindsay, H. D., Flaggs, G., DeMaggio, A. J., Hoekstra, M. F., Carr, A. M. & Bentley, N. J. (1998) EMBO J. 17, 7239–7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Girona, A., Tanaka, K., Chen, X. B., Baber, B. A., McGowan, C. H. & Russell, P. (2001) Proc. Natl. Acad. Sci. USA 98, 11289–11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao, H. & Piwnica-Worms, H. (2001) Mol. Cell. Biol. 21, 4129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastan, M. B. & Lim, D. S. (2000) Nat. Rev. Mol. Cell Biol. 1, 179–186. [DOI] [PubMed] [Google Scholar]
- 13.Siede, W., Friedberg, A. S. & Friedberg, E. C. (1993) Proc. Natl. Acad. Sci. USA 90, 7985–7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costanzo, V., Robertson, K., Ying, C. Y., Kim, E., Avvedimento, E., Gottesman, M., Grieco, D. & Gautier, J. (2000) Mol. Cell 6, 649–659. [DOI] [PubMed] [Google Scholar]
- 15.Meyn, M. S. (1995) Cancer Res. 55, 5991–6001. [PubMed] [Google Scholar]
- 16.Zhou, B. B. & Elledge, S. J. (2000) Nature 408, 433–439. [DOI] [PubMed] [Google Scholar]
- 17.Rhind, N. & Russell, P. (1998) Genetics 149, 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearsey, S. E., Montgomery, S., Labib, K. & Lindner, K. (2000) EMBO J. 19, 1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno, S., Klar, A. & Nurse, P. (1991) Methods Enzymol. 194, 795–823. [DOI] [PubMed] [Google Scholar]
- 20.Sazer, S. & Sherwood, S. W. (1990) J. Cell Sci. 97, 509–516. [DOI] [PubMed] [Google Scholar]
- 21.Muller, M., Lucchini, R. & Sogo, J. M. (2000) Mol. Cell 5, 767–777. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez, J. A., Kim, S. M. & Huberman, J. A. (1998) Exp. Cell Res. 238, 220–230. [DOI] [PubMed] [Google Scholar]
- 23.Aves, S. J., Durkacz, B. W., Carr, A. & Nurse, P. (1985) EMBO J. 4, 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka, K., Okazaki, K., Okazaki, N., Ueda, T., Sugiyama, A., Nojima, H. & Okayama, H. (1992) EMBO J. 11, 4923–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowndes, N. F., McInerny, C. J., Johnson, A. L., Fantes, P. A. & Johnston, L. H. (1992) Nature 355, 449–453. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka, K. & Okayama, H. (2000) Mol. Biol. Cell 11, 2845–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitehall, S., Stacey, P., Dawson, K. & Jones, N. (1999) Mol. Biol. Cell 10, 3705–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, S. M. & Huberman, J. A. (2001) EMBO J. 20, 6115–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurse, P. & Bissett, Y. (1981) Nature 292, 558–560. [DOI] [PubMed] [Google Scholar]
- 30.Hayles, J. & Nurse, P. (1995) EMBO J. 14, 2760–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarzov, P., Decottignies, A., Baldacci, G. & Nurse, P. (2002) EMBO J. 21, 3370–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundgren, K., Walworth, N., Booher, R., Dembski, M., Kirschner, M. & Beach, D. (1991) Cell 64, 1111–1122. [DOI] [PubMed] [Google Scholar]
- 33.O'Connell, M. J., Raleigh, J. M., Verkade, H. M. & Nurse, P. (1997) EMBO J. 16, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhind, N., Furnari, B. & Russell, P. (1997) Genes Dev. 11, 504–511. [DOI] [PubMed] [Google Scholar]
- 35.Sidorova, J. M. & Breeden, L. L. (1997) Genes Dev. 11, 3032–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolmach, L. J., Jones, R. W. & Busse, P. M. (1977) Radiat. Res. 71, 653–665. [PubMed] [Google Scholar]
- 37.Kumagai, A., Guo, Z., Emami, K. H., Wang, S. X. & Dunphy, W. G. (1998) J. Cell Biol. 142, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moser, B. A., Brondello, J. M., Baber-Furnari, B. & Russell, P. (2000) Mol. Cell. Biol. 20, 4288–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlson, C. R., Grallert, B., Stokke, T. & Boye, E. (1999) J. Cell Sci. 112, 939–946. [DOI] [PubMed] [Google Scholar]
- 40.Neecke, H., Lucchini, G. & Longhese, M. P. (1999) EMBO J. 18, 4485–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerald, J. N., Benjamin, J. M. & Kron, S. J. (2002) J. Cell Sci. 115, 1749–1757. [DOI] [PubMed] [Google Scholar]
- 42.Lee, H., Larner, J. M. & Hamlin, J. L. (1997) Proc. Natl. Acad. Sci. USA 94, 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kastan, M. B., Onyekwere, O., Sidransky, D., Vogelstein, B. & Craig, R. W. (1991) Cancer Res. 51, 6304–6311. [PubMed] [Google Scholar]
- 44.Olive, P. L., Banath, J. P. & Durand, R. E. (1990) Radiat. Res. 122, 86–94. [PubMed] [Google Scholar]
- 45.Siede, W., Friedberg, A. S., Dianova, I. & Friedberg, E. C. (1994) Genetics 138, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidorova, J. M. & Breeden, L. L. (2003) Mol. Cell. Biol. 23, 3405–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cortez, D. (July 7, 2003) J. Biol. Chem., 10.1074/jbc.M307088200.
- 48.Denison, S. H., Kafer, E. & May, G. S. (1993) Genetics 134, 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guacci, V., Koshland, D. & Strunnikov, A. (1997) Cell 91, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darwiche, N., Freeman, L. A. & Strunnikov, A. (1999) Gene 233, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou, H., McGarry, T. J., Bernal, T. & Kirschner, M. W. (1999) Science 285, 418–422. [DOI] [PubMed] [Google Scholar]
- 52.van Heemst, D., James, F., Poggeler, S., Berteaux-Lecellier, V. & Zickler, D. (1999) Cell 98, 261–271. [DOI] [PubMed] [Google Scholar]
- 53.van Heemst, D., Kafer, E., John, T., Heyting, C., van Aalderen, M. & Zickler, D. (2001) Proc. Natl. Acad. Sci. USA 98, 6267–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cha, R. S. & Kleckner, N. (2002) Science 297, 602–606. [DOI] [PubMed] [Google Scholar]
- 55.Haase, S. B. & Reed, S. I. (1999) Nature 401, 394–397. [DOI] [PubMed] [Google Scholar]
- 56.Roussel, M. R. (2000) BioEssays 22, 3–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.