Abstract
The role of bcl-2 expression in solid tumours is as yet undefined. It was, therefore, the purpose of this study to investigate expression of bcl-2 protein in 111 human breast carcinomas using immunohistochemistry and the monoclonal antibody bcl-2 124. Expression was then compared with the established indicators of prognosis and biological behaviour in malignant breast disease. No relationship could be observed between bcl-2 and node status, tumour size, differentiation, type or age at excision. However, a strong positive relationship was seen between bcl-2 and oestrogen receptor (ER), with 70 of 88 (80%) bcl-2-positive tumours being ER positive also, compared with seven of 23 (30%) bcl-2-negative tumours being ER positive (P < 0.0001). The converse was found when bcl-2 was compared with epidermal growth factor receptor (EGFR). A strong negative relationship was observed, with 26 of 88 (30%) bcl-2-positive tumours being EGFR positive, compared with 16 of 23 (70%) bcl-2-negative tumours being EGFR positive (P = 0.001), raising the possibility that bcl-2 is an ER-regulated gene. An inverse relationship was also found between bcl-2 and the oncogenes c-erbB-2 and p53. Thus, loss of bcl-2 expression in breast cancer is associated with a range of molecular markers of poor prognosis and may define part of an ER-negative, EGFR-positive phenotype.
Full text
PDF

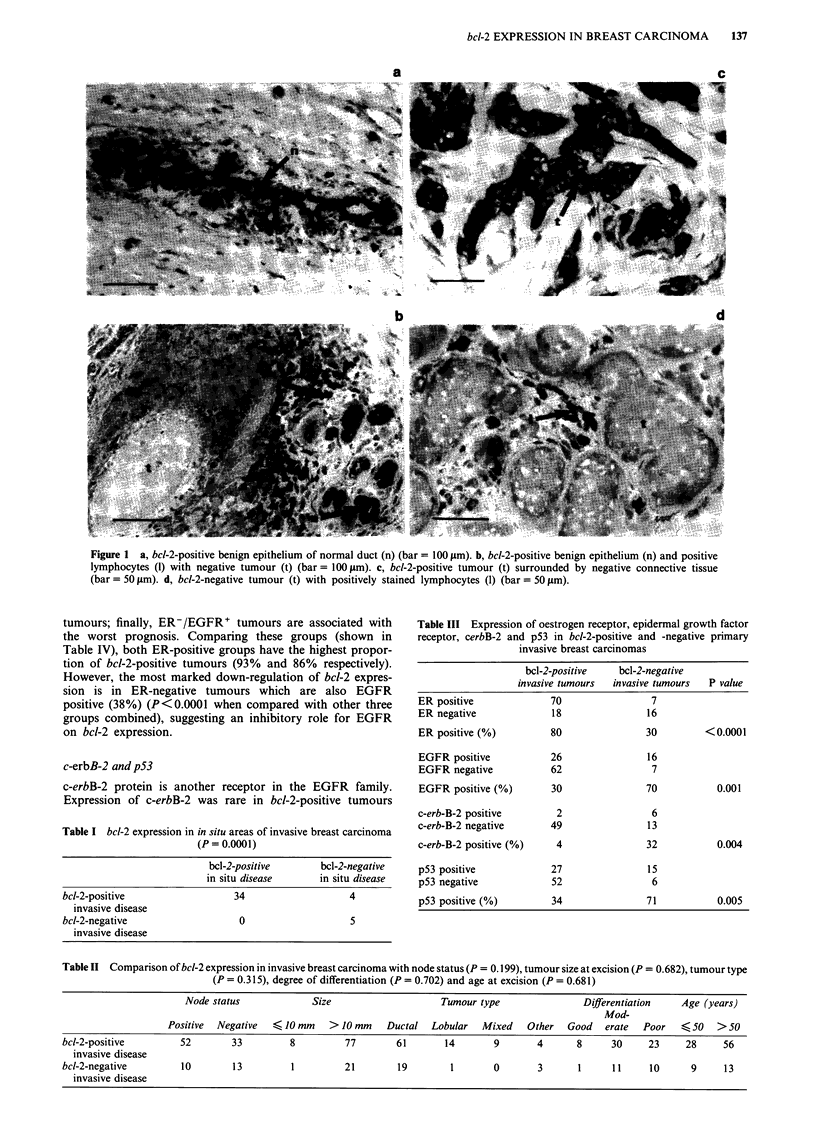


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Barnes D. M., Lammie G. A., Millis R. R., Gullick W. L., Allen D. S., Altman D. G. An immunohistochemical evaluation of c-erbB-2 expression in human breast carcinoma. Br J Cancer. 1988 Oct;58(4):448–452. doi: 10.1038/bjc.1988.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen-Levy Z., Nourse J., Cleary M. L. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989 Feb;9(2):701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Corbett I. P., Henry J. A., Angus B., Watchorn C. J., Wilkinson L., Hennessy C., Gullick W. J., Tuzi N. L., May F. E., Westley B. R. NCL-CB11, a new monoclonal antibody recognizing the internal domain of the c-erbB-2 oncogene protein effective for use on formalin-fixed, paraffin-embedded tissue. J Pathol. 1990 May;161(1):15–25. doi: 10.1002/path.1711610105. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Graninger W. B., Seto M., Boutain B., Goldman P., Korsmeyer S. J. Expression of Bcl-2 and Bcl-2-Ig fusion transcripts in normal and neoplastic cells. J Clin Invest. 1987 Nov;80(5):1512–1515. doi: 10.1172/JCI113235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Howell A., Barnes D. M., Harland R. N., Redford J., Bramwell V. H., Wilkinson M. J., Swindell R., Crowther D., Sellwood R. A. Steroid-hormone receptors and survival after first relapse in breast cancer. Lancet. 1984 Mar 17;1(8377):588–591. doi: 10.1016/s0140-6736(84)90995-4. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., Clark G. M. Prognostic factors and treatment decisions in axillary-node-negative breast cancer. N Engl J Med. 1992 Jun 25;326(26):1756–1761. doi: 10.1056/NEJM199206253262607. [DOI] [PubMed] [Google Scholar]
- Nicholson S., Sainsbury J. R., Needham G. K., Chambers P., Farndon J. R., Harris A. L. Quantitative assays of epidermal growth factor receptor in human breast cancer: cut-off points of clinical relevance. Int J Cancer. 1988 Jul 15;42(1):36–41. doi: 10.1002/ijc.2910420108. [DOI] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Pezzella F., Jones M., Ralfkiaer E., Ersbøll J., Gatter K. C., Mason D. Y. Evaluation of bcl-2 protein expression and 14;18 translocation as prognostic markers in follicular lymphoma. Br J Cancer. 1992 Jan;65(1):87–89. doi: 10.1038/bjc.1992.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzella F., Morrison H., Jones M., Gatter K. C., Lane D., Harris A. L., Mason D. Y. Immunohistochemical detection of p53 and bcl-2 proteins in non-Hodgkin's lymphoma. Histopathology. 1993 Jan;22(1):39–44. doi: 10.1111/j.1365-2559.1993.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Needham G. K., Malcolm A. J., Harris A. L. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987 Jun 20;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- Shaw P., Bovey R., Tardy S., Sahli R., Sordat B., Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ikegaki N., Croce C. M. Characterization of the protein product of bcl-2, the gene involved in human follicular lymphoma. Oncogene. 1987;2(1):3–7. [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wright C., Angus B., Nicholson S., Sainsbury J. R., Cairns J., Gullick W. J., Kelly P., Harris A. L., Horne C. H. Expression of c-erbB-2 oncoprotein: a prognostic indicator in human breast cancer. Cancer Res. 1989 Apr 15;49(8):2087–2090. [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]