Abstract
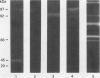
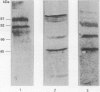
Matrix metalloproteinase (MMP) expression was investigated in patients with prostatic adenocarcinoma and benign prostatic hyperplasia (BPH). Forty-one men were studied: 26 had histologically proven prostate cancer, with 14 (54%) showing metastatic disease; 15 patients had BPH. Prostatic tissue was obtained from transurethral resection and needle core biopsies; gelatinolytic activity was determined by zymography. Seven gelatinolytic bands were detected, with molecular weights ranging from > 100 kilodalton (kDa) to 29 kDa. Nine of 14 patients (64%) with skeletal metastases had 92 kDa activity, present in only two of 12 patients (17%) with a negative bone scan, and absent in BPH. The 92 kDa gelatinolytic activity was expressed in 73% of aneuploid tumours compared with 20% of diploid tumours. A 97 kDa gelatinase was expressed in 80% of BPH samples and 23% of carcinoma patients. Enzyme bands of 72, 66 and 45 kDa were equally expressed in malignant tissue, irrespective of metastatic status, but were expressed in fewer BPH patients. The 97, 92, 66 and 45 kDa enzymes were identified as being pro-MMP-9 sequences by Western blotting, using a specific antibody directed against the pro sequence of the mature protein. MMP activity appeared to be increased in malignant prostatic tissue compared with BPH. Pro-MMP-9, in its 92 kDa form, was shown to be exclusively expressed by malignant prostatic tissue, and in particular by tumours that exhibited the aggressive and metastatic phenotype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballin M., Gomez D. E., Sinha C. C., Thorgeirsson U. P. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem Biophys Res Commun. 1988 Aug 15;154(3):832–838. doi: 10.1016/0006-291x(88)90215-x. [DOI] [PubMed] [Google Scholar]
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Bernhard E. J., Muschel R. J., Hughes E. N. Mr 92,000 gelatinase release correlates with the metastatic phenotype in transformed rat embryo cells. Cancer Res. 1990 Jul 1;50(13):3872–3877. [PubMed] [Google Scholar]
- Boag A. H., Young I. D. Immunohistochemical analysis of type IV collagenase expression in prostatic hyperplasia and adenocarcinoma. Mod Pathol. 1993 Jan;6(1):65–68. [PubMed] [Google Scholar]
- Brown P. D., Levy A. T., Margulies I. M., Liotta L. A., Stetler-Stevenson W. G. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990 Oct 1;50(19):6184–6191. [PubMed] [Google Scholar]
- Fidler I. J. Cancer metastasis. Br Med Bull. 1991 Jan;47(1):157–177. doi: 10.1093/oxfordjournals.bmb.a072453. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Hart I. R. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982 Sep 10;217(4564):998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- George N. J. Natural history of localised prostatic cancer managed by conservative therapy alone. Lancet. 1988 Mar 5;1(8584):494–497. doi: 10.1016/s0140-6736(88)91294-9. [DOI] [PubMed] [Google Scholar]
- Gittes R. F. Carcinoma of the prostate. N Engl J Med. 1991 Jan 24;324(4):236–245. doi: 10.1056/NEJM199101243240406. [DOI] [PubMed] [Google Scholar]
- Gleason D. F. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966 Mar;50(3):125–128. [PubMed] [Google Scholar]
- Gleason D. F., Mellinger G. T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974 Jan;111(1):58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- HIRST A. E., Jr, BERGMAN R. T. Carcinoma of the prostate in men 80 or more years old. Cancer. 1954 Jan;7(1):136–141. doi: 10.1002/1097-0142(195401)7:1<136::aid-cncr2820070114>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Johansson J. E., Adami H. O., Andersson S. O., Bergström R., Holmberg L., Krusemo U. B. High 10-year survival rate in patients with early, untreated prostatic cancer. JAMA. 1992 Apr 22;267(16):2191–2196. [PubMed] [Google Scholar]
- Johansson J. E., Adami H. O., Andersson S. O., Bergström R., Krusemo U. B., Kraaz W. Natural history of localised prostatic cancer. A population-based study in 223 untreated patients. Lancet. 1989 Apr 15;1(8642):799–803. doi: 10.1016/s0140-6736(89)92269-1. [DOI] [PubMed] [Google Scholar]
- Karelina T. V., Hruza G. J., Goldberg G. I., Eisen A. Z. Localization of 92-kDa type IV collagenase in human skin tumors: comparison with normal human fetal and adult skin. J Invest Dermatol. 1993 Feb;100(2):159–165. doi: 10.1111/1523-1747.ep12462791. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Kleinerman J., Catanzaro P., Rynbrandt D. Degradation of basement membrane by murine tumor cells. J Natl Cancer Inst. 1977 May;58(5):1427–1431. doi: 10.1093/jnci/58.5.1427. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Stetler-Stevenson W. G. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991 Sep 15;51(18 Suppl):5054s–5059s. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Murphy G. J., Murphy G., Reynolds J. J. The origin of matrix metalloproteinases and their familial relationships. FEBS Lett. 1991 Sep 2;289(1):4–7. doi: 10.1016/0014-5793(91)80895-a. [DOI] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Hembry R. M. Metalloproteinases and cancer invasion and metastasis. Int J Cancer. 1989 Oct 15;44(4):757–760. doi: 10.1002/ijc.2910440434. [DOI] [PubMed] [Google Scholar]
- Pajouh M. S., Nagle R. B., Breathnach R., Finch J. S., Brawer M. K., Bowden G. T. Expression of metalloproteinase genes in human prostate cancer. J Cancer Res Clin Oncol. 1991;117(2):144–150. doi: 10.1007/BF01613138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. C., Knox J. D., Navre M., Grogan T. M., Kittelson J., Nagle R. B., Bowden G. T. Expression of the metalloproteinase matrilysin in DU-145 cells increases their invasive potential in severe combined immunodeficient mice. Cancer Res. 1993 Jan 15;53(2):417–422. [PubMed] [Google Scholar]
- Pyke C., Ralfkiaer E., Huhtala P., Hurskainen T., Danø K., Tryggvason K. Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenases in human skin cancers by in situ hybridization. Cancer Res. 1992 Mar 1;52(5):1336–1341. [PubMed] [Google Scholar]
- Sato H., Kida Y., Mai M., Endo Y., Sasaki T., Tanaka J., Seiki M. Expression of genes encoding type IV collagen-degrading metalloproteinases and tissue inhibitors of metalloproteinases in various human tumor cells. Oncogene. 1992 Jan;7(1):77–83. [PubMed] [Google Scholar]
- Sato H., Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993 Feb;8(2):395–405. [PubMed] [Google Scholar]
- Stearns M. E., Wang M. Type IV collagenase (M(r) 72,000) expression in human prostate: benign and malignant tissue. Cancer Res. 1993 Feb 15;53(4):878–883. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Wacher M. P., Margulies I. M., Liotta L. A. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem. 1989 Jan 25;264(3):1353–1356. [PubMed] [Google Scholar]
- Tavares A. S., Costa J., Maia J. C. Correlation between ploidy and prognosis in prostatic carcinoma. J Urol. 1973 Apr;109(4):676–679. doi: 10.1016/s0022-5347(17)60513-5. [DOI] [PubMed] [Google Scholar]
- Tissot J. D., Hauert J., Bachmann F. Characterization of plasminogen activators from normal human breast and colon and from breast and colon carcinomas. Int J Cancer. 1984 Sep 15;34(3):295–302. doi: 10.1002/ijc.2910340302. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Whitmore W. F., Jr Natural history and staging of prostate cancer. Urol Clin North Am. 1984 May;11(2):205–220. [PubMed] [Google Scholar]
- Wilson M. J., Norris H., Kapoor D., Woodson M., Limas C., Sinha A. A. Gelatinolytic and caseinolytic proteinase activities in human prostatic secretions. J Urol. 1993 Mar;149(3):653–658. doi: 10.1016/s0022-5347(17)36173-6. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Yamagata S., Ito Y., Tanaka R., Shimizu S. Gelatinases of metastatic cell lines of murine colonic carcinoma as detected by substrate-gel electrophoresis. Biochem Biophys Res Commun. 1988 Feb 29;151(1):158–162. doi: 10.1016/0006-291x(88)90573-6. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Tanaka R., Ito Y., Shimizu S. Gelatinases of murine metastatic tumor cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):228–234. doi: 10.1016/s0006-291x(89)80202-5. [DOI] [PubMed] [Google Scholar]