Abstract
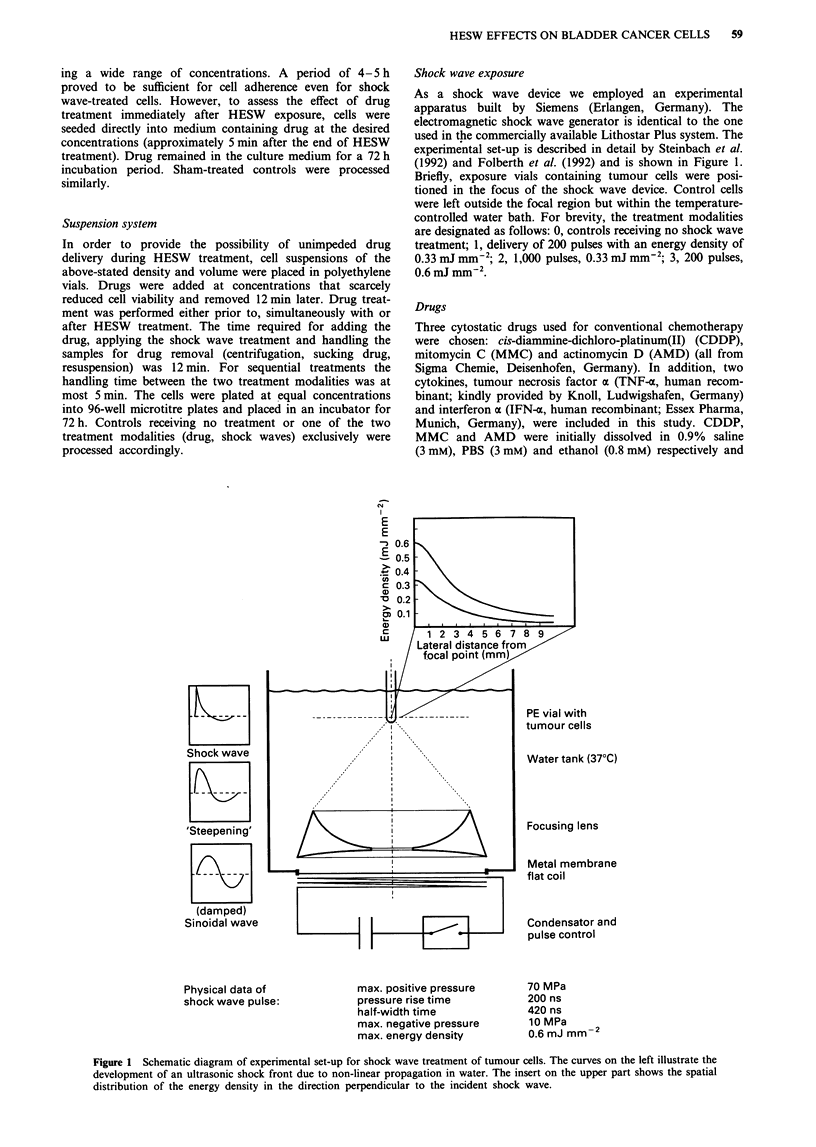
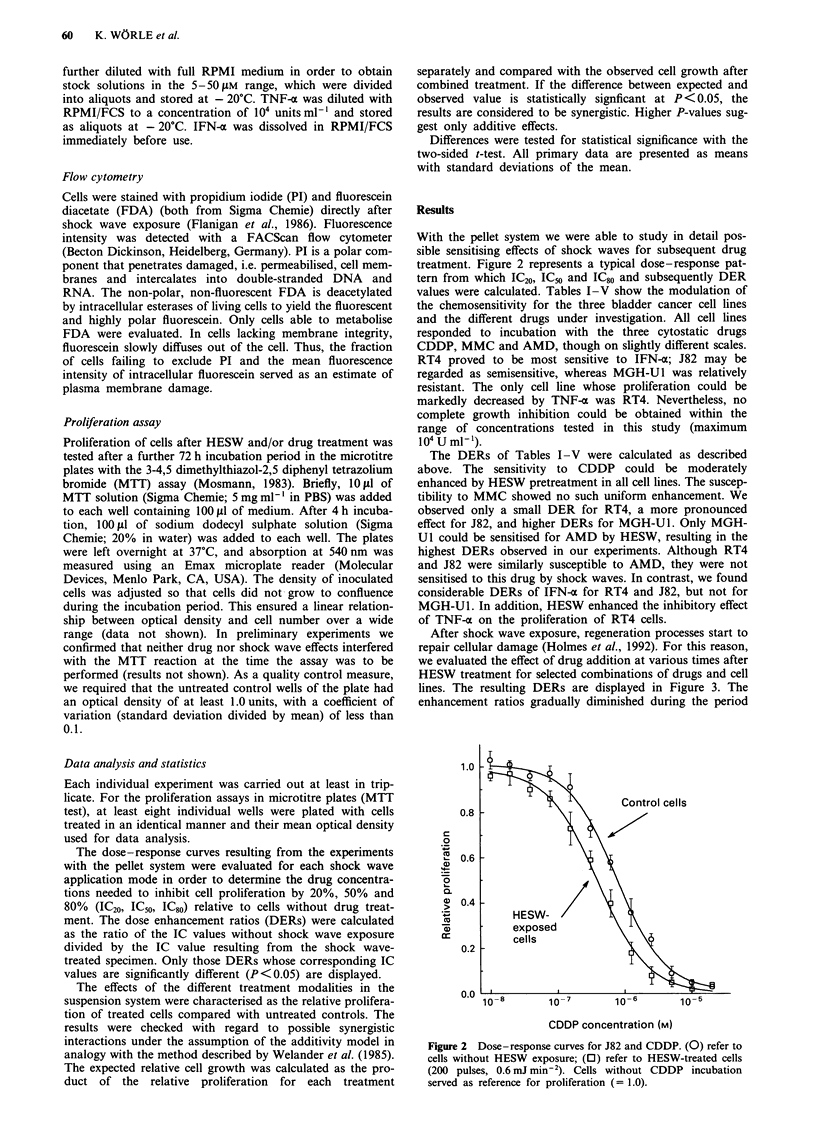
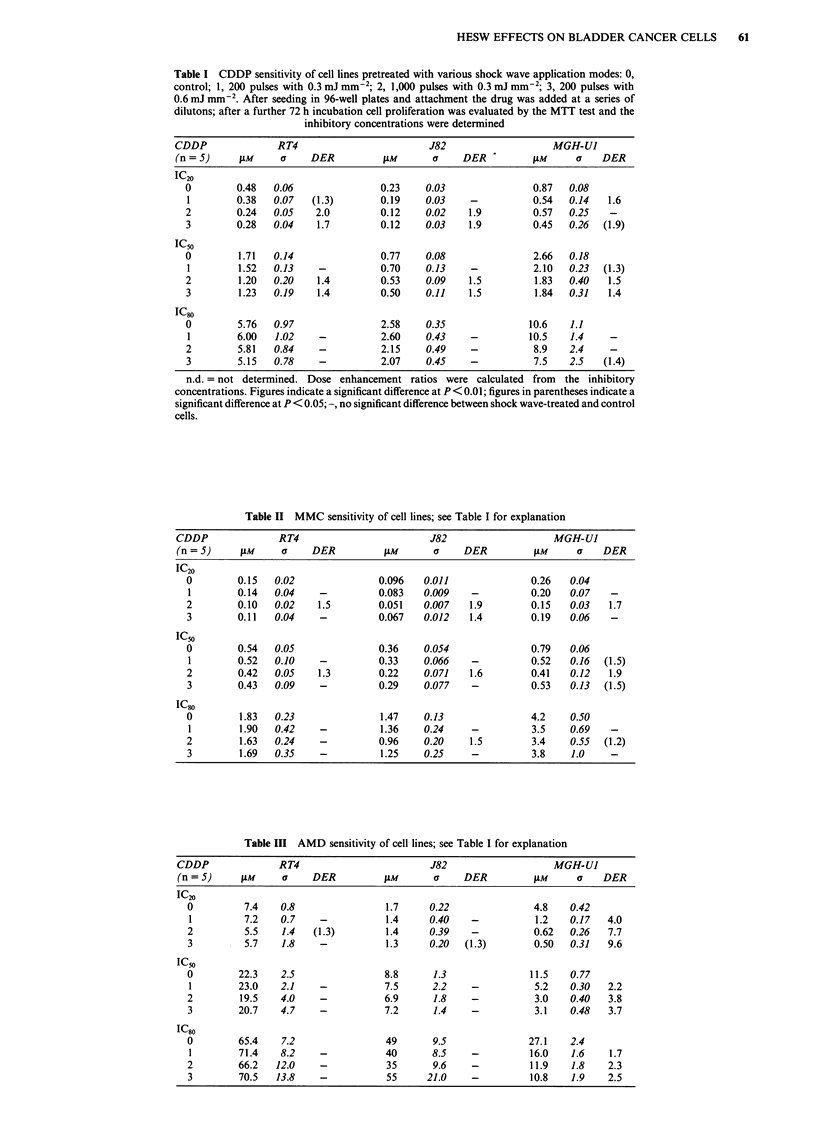
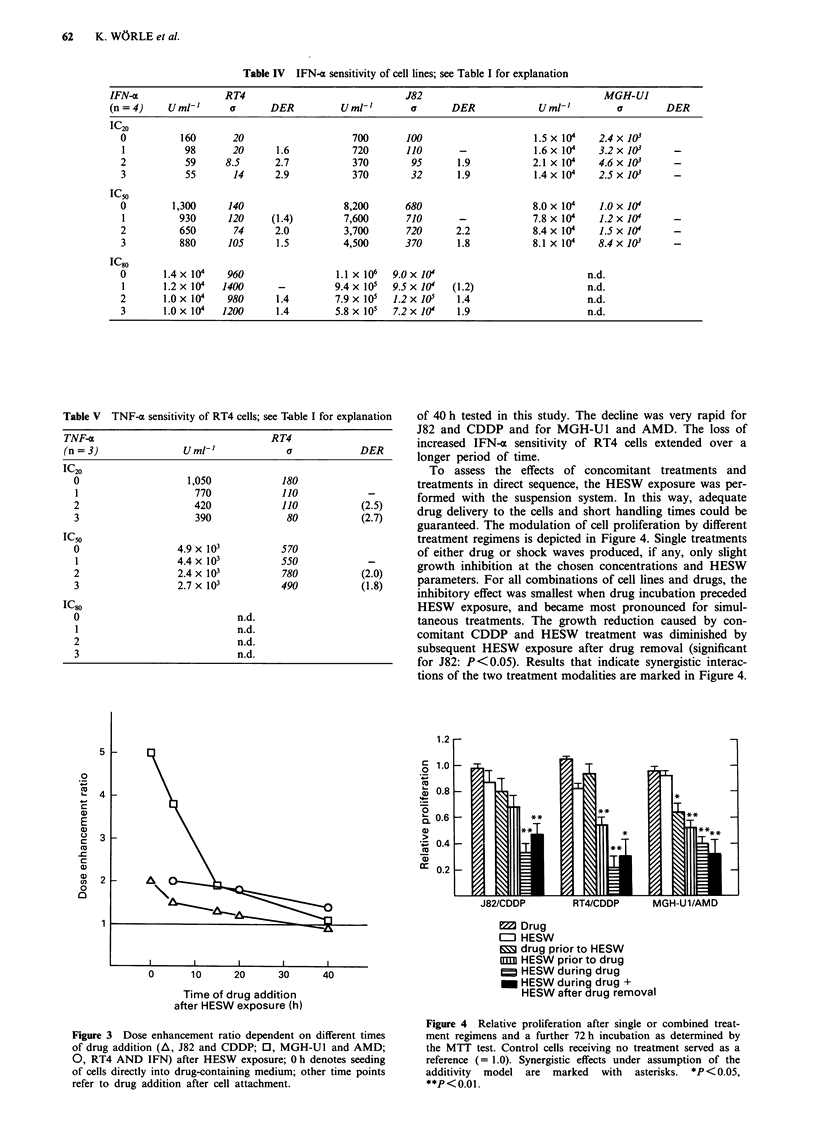
The effects of shock waves generated by an experimental Siemens lithotripter in combination with cytostatic drugs or cytokines on several bladder cancer cell lines were examined in vitro. Proliferation after treatment was determined with the 3-4,5-dimethylthiazol-2,5 diphenyl tetrazolium bromide assay. Dose enhancement ratios were calculated for each drug and each shock wave application mode in order to characterise the sensitising effect of shock wave pretreatment. The influence of the time between shock wave and drug treatment as well as the effects of different sequences of shock wave and drug treatment or concomitant treatment were assessed for selected combinations of cell lines and drugs. It was found that shock wave treatment could render certain cell lines more susceptible to subsequent cis-platinum, mitomycin C or actinomycin D incubation. Cell lines sensitive to tumour necrosis factor alpha or interferon alpha were further sensitised to these cytokines by shock wave pretreatment. The enhanced sensitivity to cis-platinum and actinomycin D decreased rapidly during the first hours after shock wave treatment. The antiproliferative effect was most pronounced after concomitant shock wave and drug treatment. The sensitisation to interferon alpha diminishes more slowly after shock wave exposure. From the results presented in this study it is concluded that transient shock wave-induced permeabilisation of cell membrane not only enhances drug efficiency, but also causes damage to cell organelles and alterations in cellular metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anghileri L. J., Robert J. Effects of tumor necrosis factor on tumor cell plasma membrane permeability. Tumori. 1987 Jun 30;73(3):269–271. doi: 10.1177/030089168707300310. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Corry P. M., Drewinko B. In vitro thermochemotherapy of human colon cancer cells with cis-dichlorodiammineplatinum(II) and mitomycin C. Cancer Res. 1980 Apr;40(4):1165–1168. [PubMed] [Google Scholar]
- Baron S., Tyring S. K., Fleischmann W. R., Jr, Coppenhaver D. H., Niesel D. W., Klimpel G. R., Stanton G. J., Hughes T. K. The interferons. Mechanisms of action and clinical applications. JAMA. 1991 Sep 11;266(10):1375–1383. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- Berens M. E., Welander C. E., Griffin A. S., McCullough D. L. Effect of acoustic shock waves on clonogenic growth and drug sensitivity of human tumor cells in vitro. J Urol. 1989 Oct;142(4):1090–1094. doi: 10.1016/s0022-5347(17)39002-x. [DOI] [PubMed] [Google Scholar]
- Bräuner T., Brümmer F., Hülser D. F. Histopathology of shock wave treated tumor cell suspensions and multicell tumor spheroids. Ultrasound Med Biol. 1989;15(5):451–460. doi: 10.1016/0301-5629(89)90098-7. [DOI] [PubMed] [Google Scholar]
- Ellwart J. W., Brettel H., Kober L. O. Cell membrane damage by ultrasound at different cell concentrations. Ultrasound Med Biol. 1988;14(1):43–50. doi: 10.1016/0301-5629(88)90162-7. [DOI] [PubMed] [Google Scholar]
- Fahnestock M., Rimer V. G., Yamawaki R. M., Ross P., Edmonds P. D. Effects of ultrasound exposure in vitro on neuroblastoma cell membranes. Ultrasound Med Biol. 1989;15(2):133–144. doi: 10.1016/0301-5629(89)90162-2. [DOI] [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991 Jul 22;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Flanigan R. C., Pavlik E. J., Van Nagell J. R., Keaton K., Kenady D. E. Proliferation, esterase activity, and propidium iodide exclusion in urologic tumor cells after in vitro exposure to chemotherapeutic agents. J Urol. 1986 May;135(5):1091–1100. doi: 10.1016/s0022-5347(17)45982-9. [DOI] [PubMed] [Google Scholar]
- Flitter W. D., Mason R. P. The enzymatic reduction of actinomycin D to a free radical species. Arch Biochem Biophys. 1988 Dec;267(2):632–639. doi: 10.1016/0003-9861(88)90071-9. [DOI] [PubMed] [Google Scholar]
- Folberth W., Köhler G., Rohwedder A., Matura E. Pressure distribution and energy flow in the focal region of two different electromagnetic shock wave sources. J Stone Dis. 1992 Jan;4(1):1–7. [PubMed] [Google Scholar]
- Gambihler S., Delius M. In vitro interaction of lithotripter shock waves and cytotoxic drugs. Br J Cancer. 1992 Jul;66(1):69–73. doi: 10.1038/bjc.1992.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambihler S., Delius M. Transient increase in membrane permeability of L1210 cells upon exposure to lithotripter shock waves in vitro. Naturwissenschaften. 1992 Jul;79(7):328–329. doi: 10.1007/BF01138714. [DOI] [PubMed] [Google Scholar]
- Hecquet B., Leroy A., Lefebvre J. L., Peyrat J. P., Adenis L. Uptake of platinum compounds in human tumors. In vitro study. Bull Cancer. 1986;73(5):535–541. [PubMed] [Google Scholar]
- Holmes R. P., Yeaman L. D., Taylor R. G., McCullough D. L. Altered neutrophil permeability following shock wave exposure in vitro. J Urol. 1992 Mar;147(3):733–737. doi: 10.1016/s0022-5347(17)37368-8. [DOI] [PubMed] [Google Scholar]
- Holmes R. P., Yeaman L. I., Li W. J., Hart L. J., Wallen C. A., Woodruff R. D., McCullough D. L. The combined effects of shock waves and cisplatin therapy on rat prostate tumors. J Urol. 1990 Jul;144(1):159–163. doi: 10.1016/s0022-5347(17)39401-6. [DOI] [PubMed] [Google Scholar]
- Hoshi S., Orikasa S., Kuwahara M., Suzuki K., Shirai S., Yoshikawa K., Nose M. Shock wave and THP-adriamycin for treatment of rabbit's bladder cancer. Jpn J Cancer Res. 1992 Mar;83(3):248–250. doi: 10.1111/j.1349-7006.1992.tb00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iro H., Schneider H. T., Födra C., Waitz G., Nitsche N., Heinritz H. H., Benninger J., Ell C. Shockwave lithotripsy of salivary duct stones. Lancet. 1992 May 30;339(8805):1333–1336. doi: 10.1016/0140-6736(92)91968-e. [DOI] [PubMed] [Google Scholar]
- Kober L. O., Ellwart J. W., Brettel H. Effect of the pulse length of ultrasound on cell membrane damage in vitro. J Acoust Soc Am. 1989 Jul;86(1):6–7. doi: 10.1121/1.398222. [DOI] [PubMed] [Google Scholar]
- Kohri K., Uemura T., Iguchi M., Kurita T. Effect of high energy shock waves on tumor cells. Urol Res. 1990;18(2):101–105. doi: 10.1007/BF00302468. [DOI] [PubMed] [Google Scholar]
- Komatsu K., Miller R. C., Hall E. J. The oncogenic potential of a combination of hyperthermia and chemotherapy agents. Br J Cancer. 1988 Jan;57(1):59–63. doi: 10.1038/bjc.1988.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. E., Smith P., Cockett A. T. Influence of high-energy shock waves and cisplatin on antitumor effect in murine bladder cancer. Urology. 1990 Nov;36(5):440–444. doi: 10.1016/s0090-4295(90)80292-u. [DOI] [PubMed] [Google Scholar]
- Lin C. W., Lin J. C., Prout G. R., Jr Establishment and characterization of four human bladder tumor cell lines and sublines with different degrees of malignancy. Cancer Res. 1985 Oct;45(10):5070–5079. [PubMed] [Google Scholar]
- Lingeman J. E., McAteer J. A., Kempson S. A., Evan A. P. Bioeffects of extracorporeal shock-wave lithotripsy. Strategy for research and treatment. Urol Clin North Am. 1988 Aug;15(3):507–514. [PubMed] [Google Scholar]
- Melvik J. E., Dornish J. M., Pettersen E. O. The binding of cis-dichlorodiammineplatinum(II) to extracellular and intracellular compounds in relation to drug uptake and cytotoxicity in vitro. Br J Cancer. 1992 Aug;66(2):260–265. doi: 10.1038/bjc.1992.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvik J. E., Pettersen E. O., Gordon P. B., Seglen P. O. Increase in cis-dichlorodiammineplatinum (II) cytotoxicity upon reversible electropermeabilization of the plasma membrane in cultured human NHIK 3025 cells. Eur J Cancer Clin Oncol. 1986 Dec;22(12):1523–1530. doi: 10.1016/0277-5379(86)90090-8. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- O'Toole C., Price Z. H., Ohnuki Y., Unsgaard B. Ultrastructure, karyology and immunology of a cell line originated from a human transitional-cell carcinoma. Br J Cancer. 1978 Jul;38(1):64–76. doi: 10.1038/bjc.1978.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhof G. O., Smits G. A., de Ruyter A. E., Schalken J. A., Debruyne F. M. Effects of high-energy shock waves combined with biological response modifiers in different human kidney cancer xenografts. Ultrasound Med Biol. 1991;17(4):391–399. doi: 10.1016/0301-5629(91)90139-n. [DOI] [PubMed] [Google Scholar]
- Orlowski S., Belehradek J., Jr, Paoletti C., Mir L. M. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol. 1988 Dec 15;37(24):4727–4733. doi: 10.1016/0006-2952(88)90344-9. [DOI] [PubMed] [Google Scholar]
- Randazzo R. F., Chaussy C. G., Fuchs G. J., Bhuta S. M., Lovrekovich H., deKernion J. B. The in vitro and in vivo effects of extracorporeal shock waves on malignant cells. Urol Res. 1988;16(6):419–426. doi: 10.1007/BF00280022. [DOI] [PubMed] [Google Scholar]
- Rigby C. C., Franks L. M. A human tissue culture cell line from a transitional cell tumour of the urinary bladder: growth, chromosone pattern and ultrastructure. Br J Cancer. 1970 Dec;24(4):746–754. doi: 10.1038/bjc.1970.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo P., Mies C., Huryk R., Heston W. D., Fair W. R. Histopathologic and ultrastructural correlates of tumor growth suppression by high energy shock waves. J Urol. 1987 Feb;137(2):338–341. doi: 10.1016/s0022-5347(17)44018-3. [DOI] [PubMed] [Google Scholar]
- Sackmann M., Paumgartner G. Biliary lithotripsy by extracorporeally generated shock waves. Recenti Prog Med. 1992 Jul-Aug;83(7-8):400–406. [PubMed] [Google Scholar]
- Schütze S., Machleidt T., Krönke M. Mechanisms of tumor necrosis factor action. Semin Oncol. 1992 Apr;19(2 Suppl 4):16–24. [PubMed] [Google Scholar]
- Simon J., Corbusier A., Mendes Leal A., Van den Bossche M., Wespes E., Van Regemorter G., Schulman C. C. Extracorporeal shock wave lithotripsy for urinary stone disease: clinical experience with the electromagnetic lithotriptor 'Lithostar'. Eur Urol. 1989;16(1):7–11. doi: 10.1159/000471519. [DOI] [PubMed] [Google Scholar]
- Smits G. A., Heerschap A., Oosterhof G. O., Ruys J. H., Hilbers C. W., Debruyne F. M., Schalken J. A. Early metabolic response to high energy shock waves in a human tumor kidney xenograft monitored by 31P magnetic resonance spectroscopy. Ultrasound Med Biol. 1991;17(8):791–801. doi: 10.1016/0301-5629(91)90162-p. [DOI] [PubMed] [Google Scholar]
- Stalc A., Sentjurc M., Sersa G., Novaković S. The influence of TNF on the membrane fluidity of tumor cells. Cancer Lett. 1992 Aug 31;65(3):183–187. doi: 10.1016/0304-3835(92)90230-s. [DOI] [PubMed] [Google Scholar]
- Steinbach P., Hofstaedter F., Nicolai H., Roessler W., Wieland W. Determination of the energy-dependent extent of vascular damage caused by high-energy shock waves in an umbilical cord model. Urol Res. 1993;21(4):279–282. doi: 10.1007/BF00307711. [DOI] [PubMed] [Google Scholar]
- Steinbach P., Hofstädter F., Nicolai H., Rössler W., Wieland W. In vitro investigations on cellular damage induced by high energy shock waves. Ultrasound Med Biol. 1992;18(8):691–699. doi: 10.1016/0301-5629(92)90120-y. [DOI] [PubMed] [Google Scholar]
- Troger V., Fischel J. L., Formento P., Gioanni J., Milano G. Effects of prolonged exposure to cisplatin on cytotoxicity and intracellular drug concentration. Eur J Cancer. 1992;28(1):82–86. doi: 10.1016/0959-8049(92)90391-e. [DOI] [PubMed] [Google Scholar]
- Wallner K. E., Banda M., Li G. C. Hyperthermic enhancement of cell killing by mitomycin C in mitomycin C-resistant Chinese hamster ovary cells. Cancer Res. 1987 Mar 1;47(5):1308–1312. [PubMed] [Google Scholar]
- Warlters A., Morris D. L., Cameron-Strange A., Lynch W. Effect of electrohydraulic and extracorporeal shock waves on gastrointestinal cancer cells and their response to cytotoxic agents. Gut. 1992 Jun;33(6):791–793. doi: 10.1136/gut.33.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D. What's new about tumor necrosis factors? Report on the fourth TNF International Congress. Eur Cytokine Netw. 1992 May-Jun;3(3):347–351. [PubMed] [Google Scholar]
- Welander C. E., Morgan T. M., Homesley H. D., Trotta P. P., Spiegel R. J. Combined recombinant human interferon alpha 2 and cytotoxic agents studied in a clonogenic assay. Int J Cancer. 1985 Jun 15;35(6):721–729. doi: 10.1002/ijc.2910350605. [DOI] [PubMed] [Google Scholar]
- Wilmer A., Gambihler S., Delius M., Brendel W. In vitro cytotoxic activity of lithotripter shock waves combined with adriamycin or with cisplatin on L1210 mouse leukemia cells. J Cancer Res Clin Oncol. 1989;115(3):229–234. doi: 10.1007/BF00391694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D. S., Chen A., Su C. J., Chang S. Y., Ma C. P., Chu T. M. Effects of high-energy shock waves on murine renal cell carcinoma. Urology. 1991 Dec;38(6):571–576. doi: 10.1016/0090-4295(91)80183-8. [DOI] [PubMed] [Google Scholar]
- Yu F. L., Bender W. Actinomycin D binding in vitro: active chromatin preferred. Biochem Int. 1990;20(4):807–815. [PubMed] [Google Scholar]
- Yung B. Y., Bor A. M., Chan P. K. Short exposure to actinomycin D induces "reversible" translocation of protein B23 as well as "reversible" inhibition of cell growth and RNA synthesis in HeLa cells. Cancer Res. 1990 Sep 15;50(18):5987–5991. [PubMed] [Google Scholar]
- Ziegler W., Birkenfeld P., Trott K. R. The effect of combined treatment of HeLa cells with actinomycin D and radiation upon survival and recovery from radiation damage. Radiother Oncol. 1987 Oct;10(2):141–148. doi: 10.1016/s0167-8140(87)80056-7. [DOI] [PubMed] [Google Scholar]



