Abstract
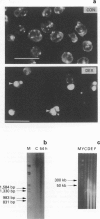
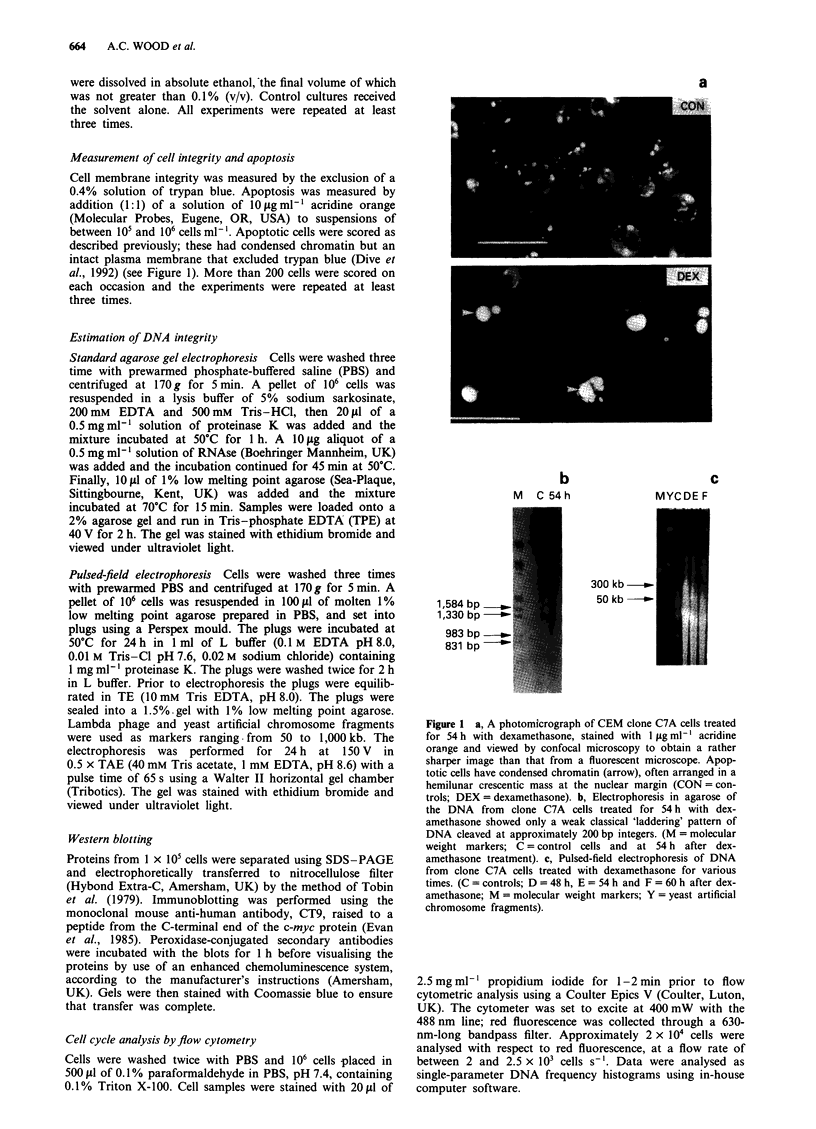
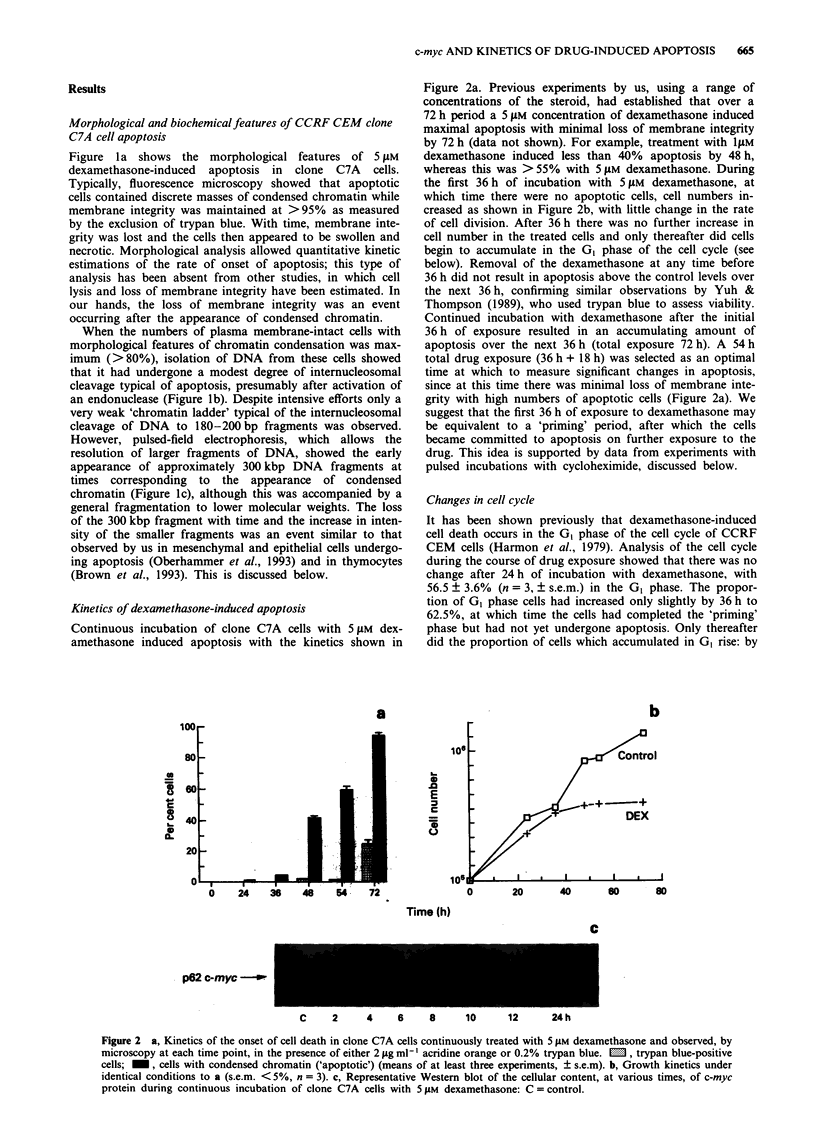
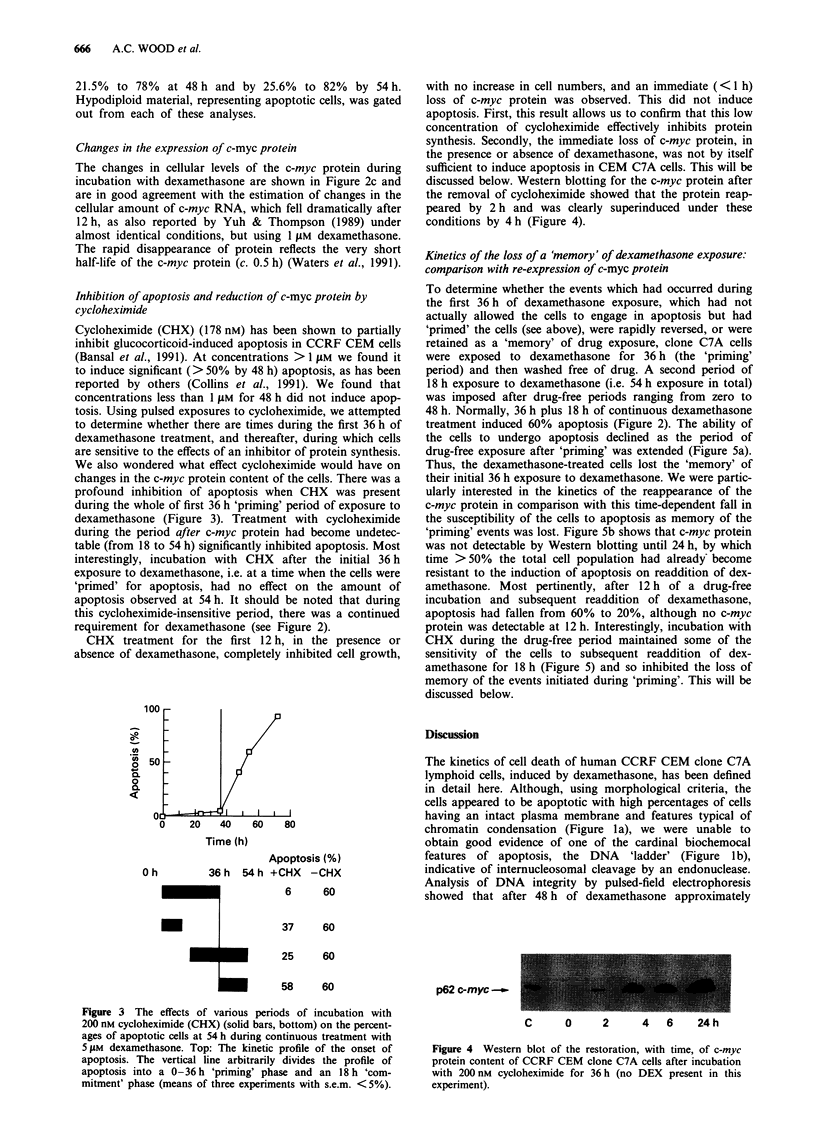
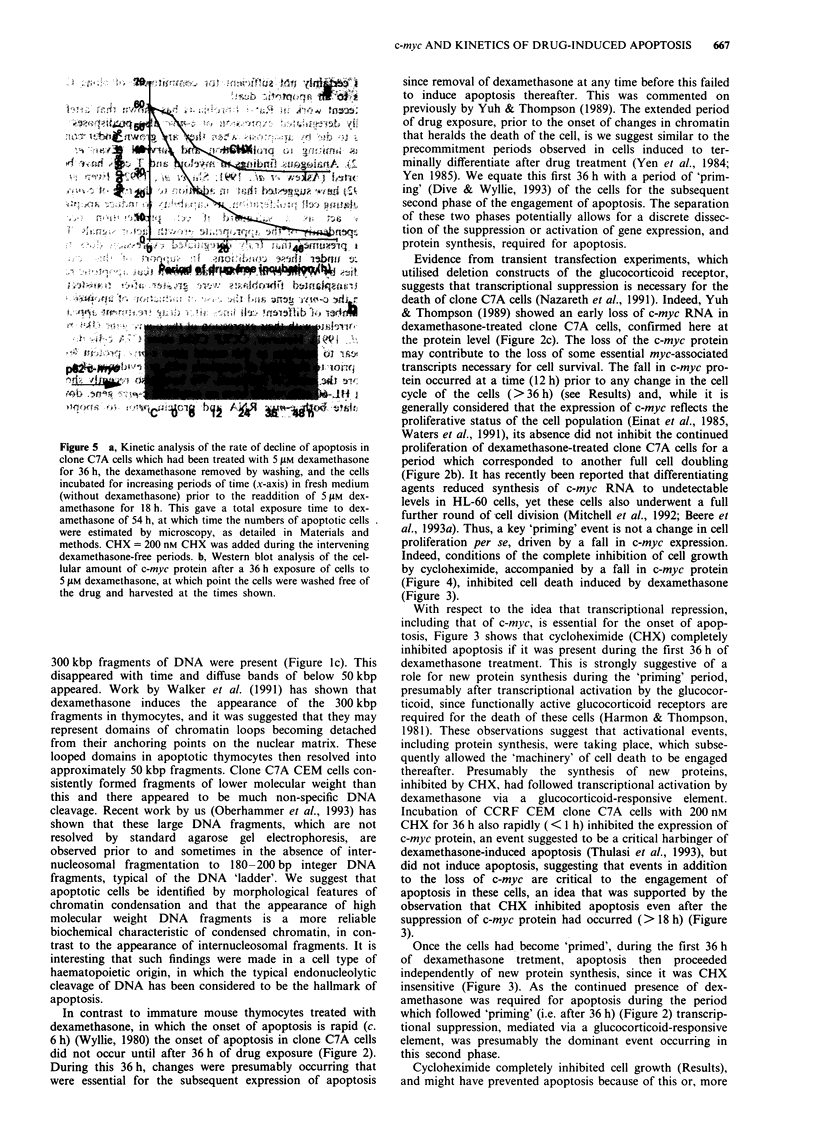
The kinetics of dexamethasone-induced death of CCRF CEM clone C7A human lymphoblastic leukaemia cells was determined with respect to changes in the expression of the c-myc protein. Cell death was characterised as being by apoptosis: cells with an intact plasma membrane had condensed chromatin and were characterised as having approximately 300 kbp fragments when DNA integrity was analysed by pulsed-field electrophoresis. Onset of apoptosis required a minimum of 36 h exposure to 5 microM dexamethasone; before this time no apoptotic cells were observed. This 36 h incubation period appeared to be necessary to prime the cells for subsequent death by apoptosis. In the continued presence of dexamethasone the percentage of apoptotic cells increased to 60% apoptotic cells by 54 h. Investigation of changes in c-myc protein showed that it was undetectable after 12 h of incubation with dexamethasone, although cells were not committed to die at this time. Cells were treated with dexamethasone for 54 h and for various pulsed periods with a non-toxic concentration of cycloheximide (200 nM). When cycloheximide was present during the first 36 h priming period of dexamethasone treatment, there was an immediate loss of c-myc protein and apoptosis at 54 h was completely inhibited. In contrast, there was no inhibition of apoptosis when dexamethasone-treated cells were incubated with an 18 h pulse of cycloheximide added after 36 h. Cells exposed to dexamethasone for 36 h ('primed') were given various periods of dexamethasone-free incubation before readdition of dexamethasone for a further 18 h. The longer the cells were free of drug after priming, the less susceptible they became to apoptosis, suggesting a slow decay of their 'memory' of the initial 36 h period of exposure. Cycloheximide inhibited the decay of this memory. Removal of dexamethasone after a 36 h exposure was characterised by a subsequent 24 h suppression of c-myc protein expression. Despite this, 90% of cells became refractory to apoptosis before the reappearance of c-myc protein. The evidence does not support the hypothesis that changes in c-myc expression are required for the engagement of apoptosis of CEM cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Bansal N., Houle A., Melnykovych G. Apoptosis: mode of cell death induced in T cell leukemia lines by dexamethasone and other agents. FASEB J. 1991 Feb;5(2):211–216. doi: 10.1096/fasebj.5.2.2004665. [DOI] [PubMed] [Google Scholar]
- Beere H. M., Morimoto R. I., Hickman J. A. Investigations of mechanisms of drug-induced changes in gene expression: N-methylformamide-induced changes in synthesis of the M(r) 72,000 constitutive heat shock protein during commitment of HL-60 cells to granulocyte differentiation. Cancer Res. 1993 Jul 1;53(13):3034–3039. [PubMed] [Google Scholar]
- Bertrand R., Sarang M., Jenkin J., Kerrigan D., Pommier Y. Differential induction of secondary DNA fragmentation by topoisomerase II inhibitors in human tumor cell lines with amplified c-myc expression. Cancer Res. 1991 Dec 1;51(23 Pt 1):6280–6285. [PubMed] [Google Scholar]
- Brown D. G., Sun X. M., Cohen G. M. Dexamethasone-induced apoptosis involves cleavage of DNA to large fragments prior to internucleosomal fragmentation. J Biol Chem. 1993 Feb 15;268(5):3037–3039. [PubMed] [Google Scholar]
- Cole M. D. Myc meets its Max. Cell. 1991 May 31;65(5):715–716. doi: 10.1016/0092-8674(91)90377-b. [DOI] [PubMed] [Google Scholar]
- Collins R. J., Harmon B. V., Souvlis T., Pope J. H., Kerr J. F. Effects of cycloheximide on B-chronic lymphocytic leukaemic and normal lymphocytes in vitro: induction of apoptosis. Br J Cancer. 1991 Sep;64(3):518–522. doi: 10.1038/bjc.1991.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dive C., Gregory C. D., Phipps D. J., Evans D. L., Milner A. E., Wyllie A. H. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim Biophys Acta. 1992 Feb 3;1133(3):275–285. doi: 10.1016/0167-4889(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schirm S., Bishop J. M. The MYC protein activates transcription of the alpha-prothymosin gene. EMBO J. 1991 Jan;10(1):133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. doi: 10.1038/313597a0. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Harmon J. M., Norman M. R., Fowlkes B. J., Thompson E. B. Dexamethasone induces irreversible G1 arrest and death of a human lymphoid cell line. J Cell Physiol. 1979 Feb;98(2):267–278. doi: 10.1002/jcp.1040980203. [DOI] [PubMed] [Google Scholar]
- Harmon J. M., Thompson E. B. Isolation and characterization of dexamethasone-resistant mutants from human lymphoid cell line CEM-C7. Mol Cell Biol. 1981 Jun;1(6):512–521. doi: 10.1128/mcb.1.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J. A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992 Sep;11(2):121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Mitchell L. S., Neill R. A., Birnie G. D. Temporal relationships between induced changes in c-myc mRNA abundance, proliferation, and differentiation in HL60 cells. Differentiation. 1992 Mar;49(2):119–125. doi: 10.1111/j.1432-0436.1992.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Nazareth L. V., Harbour D. V., Thompson E. B. Mapping the human glucocorticoid receptor for leukemic cell death. J Biol Chem. 1991 Jul 15;266(20):12976–12980. [PubMed] [Google Scholar]
- Norman M. R., Thompson E. B. Characterization of a glucocorticoid-sensitive human lymphoid cell line. Cancer Res. 1977 Oct;37(10):3785–3791. [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. P., Cohen J. J. Identification of genes involved in programmed cell death. Cancer Metastasis Rev. 1992 Sep;11(2):149–156. doi: 10.1007/BF00048061. [DOI] [PubMed] [Google Scholar]
- Shi Y., Glynn J. M., Guilbert L. J., Cotter T. G., Bissonnette R. P., Green D. R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992 Jul 10;257(5067):212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- Thulasi R., Harbour D. V., Thompson E. B. Suppression of c-myc is a critical step in glucocorticoid-induced human leukemic cell lysis. J Biol Chem. 1993 Aug 25;268(24):18306–18312. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. R., Smith C., Youdale T., Leblanc J., Whitfield J. F., Sikorska M. Topoisomerase II-reactive chemotherapeutic drugs induce apoptosis in thymocytes. Cancer Res. 1991 Feb 15;51(4):1078–1085. [PubMed] [Google Scholar]
- Waters C. M., Littlewood T. D., Hancock D. C., Moore J. P., Evan G. I. c-myc protein expression in untransformed fibroblasts. Oncogene. 1991 May;6(5):797–805. [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Rose K. A., Morris R. G., Steel C. M., Foster E., Spandidos D. A. Rodent fibroblast tumours expressing human myc and ras genes: growth, metastasis and endogenous oncogene expression. Br J Cancer. 1987 Sep;56(3):251–259. doi: 10.1038/bjc.1987.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen A. Control of HL-60 myeloid differentiation. Evidence of uncoupled growth and differentiation control, S-phase specificity, and two-step regulation. Exp Cell Res. 1985 Jan;156(1):198–212. doi: 10.1016/0014-4827(85)90274-5. [DOI] [PubMed] [Google Scholar]
- Yen A., Reece S. L., Albright K. L. Dependence of HL-60 myeloid cell differentiation on continuous and split retinoic acid exposures: precommitment memory associated with altered nuclear structure. J Cell Physiol. 1984 Mar;118(3):277–286. doi: 10.1002/jcp.1041180310. [DOI] [PubMed] [Google Scholar]
- Yuh Y. S., Thompson E. B. Glucocorticoid effect on oncogene/growth gene expression in human T lymphoblastic leukemic cell line CCRF-CEM. Specific c-myc mRNA suppression by dexamethasone. J Biol Chem. 1989 Jun 25;264(18):10904–10910. [PubMed] [Google Scholar]