Abstract
Fossil spores of the dung fungus Sporormiella spp. in sediment cores from throughout Madagascar provide new information concerning megafaunal extinction and the introduction of livestock. Sporormiella percentages are very high in prehuman southwest Madagascar, but at the site with best stratigraphic resolution the spore declines sharply by ≈1,720 yr B.P. (radiocarbon years ago). Within a few centuries there is a concomitant rise in microscopic charcoal that probably represents human transformation of the local environment. Reduced megaherbivore biomass in wooded savannas may have resulted in increased plant biomass and more severe fires. Some now-extinct taxa persisted locally for a millennium or more after the inferred megafaunal decline. Sites in closed humid forests of northwest Madagascar and a montane ericoid formation of the central highlands show only low to moderate Sporormiella percentages before humans. A subsequent rise in spore concentrations, thought to be evidence for livestock proliferation, occurs earliest at Amparihibe in the northwest at ≈1,130 yr B.P.
Fossil spores of the dung fungus Sporormiella spp. have been shown in western North America to serve as a reliable proxy for megafaunal biomass in late Pleistocene sediments. These spores decline rapidly at the end of the Pleistocene at the approximate time of megafaunal extinctions and increase again in sediments of recent centuries after livestock introduction (1, 2). We present here application of the technique described in refs. 1 and 2 to the study of late Holocene extinctions on the island of Madagascar. Sediment cores from throughout the island contain these spores, and stratigraphic trends offer a way to produce a chronology for megafaunal decline and livestock introductions.
Research over the last two decades has clarified many aspects of this remarkable ecological catastrophe (3–5), which eliminated virtually the entire endemic megafauna including the giant lemurs, elephant birds, pygmy hippopotami, and giant tortoises. Four independent lines of stratigraphic evidence are consistent with the beginning of a human presence on the island at least by ≈2,000 radiocarbon years before present (yr B.P.): (i) dates on human-modified bones of extinct animals (6); (ii) pollen of prehistorically introduced plants (7); (iii) a large spike of charcoal particles in lake and bog sediments (5); and (iv) pollen evidence for a decline in forest and increase in grasses and ruderal herbs (8). Studies of the Malagasy language also show a separation from its closest surviving linguistic relatives in the highlands of Borneo approximately two millennia ago, although divergence could have begun before proto-Malagasy speakers departed from Indonesia (9). Integrated multidisciplinary analyses of rich late Holocene fossil sites with accelerator mass spectrometry dating and close stratigraphic control show that, whereas some megafaunal taxa seem to decline rapidly after human arrival, others persisted at some sites for a millennium or more after first evidence for humans (5, 10–12). Evidence for human-caused landscape transformation (charcoal, pollen, and archaeology) (5) suggests that settlement began in the semiarid southwest of the island and spread to the other coasts and into the interior over the next 13 centuries. Remaining eastern and northwestern low-elevation humid forests and patches of montane forest and ericoid bushland at high elevations in the remote interior have been penetrated relatively slowly, with much humid forest conversion occurring since approximately anno Domini (A.D.) 1950 (13).
Pollen studies from sites throughout the island show distinct vegetation changes at many key climatic transitions of the last 35,000 yr B.P. (4, 14). Sites in the interior highlands, some associated with very rich Holocene megafaunal deposits, show a vegetation before inferred human arrival composed of a species-rich mosaic of wooded grassland, riverine and groundwater forests, and perhaps other formations lacking a close modern analogue (15). Microscopic charcoal particles in sediments show that fire was prevalent throughout the late Quaternary in these formations, but an increase to levels well above the moderate to high background charcoal probably signals human arrival locally. The interior today is almost exclusively a depauperate steppe grassland, much of which is burned annually.
The more arid southwestern part of the island, another region rich in prehistoric fossil sites of the late Holocene, had more forest than today in mid-Holocene times, but climatic aridity beginning approximately four millennia ago shifted the vegetation balance over the following millennium to a more dry-adapted mosaic of dry forest, palm savanna, and spiny bushlands dominated by giant succulents such as the endemic Didiereaceae (8). When humans arrive there approximately two millennia ago, the megafauna are still present, but the pollen record shows that palm savanna and spiny bushland have expanded into areas that were formerly dry forest. Charcoal results indicate that, after human arrival, burning greatly increased for a few centuries and then declined to moderate levels (16). As in the interior, subsequent changes, presumably mostly anthropogenic, include the decline of trees associated with closed formations and an increase in grasses and other open-country, fire-tolerant species. Dated late occurrences of now-extinct megafauna show survivals of a few giant lemur species to perhaps the 17th century or later in the most remote parts of this region (10), and legends of fantastic animals collected in recent centuries in some cases agree in detail with scientific reconstructions of the pygmy hippos and large extinct lemurs (17, 18).
Evidence from Sporormiella
Additional details concerning this transformation of the world's fourth largest island are needed, especially concerning the habitat preferences of the extinct creatures and the timing of their decline in relation to late Holocene climate change and a battery of human activities that includes fire, hunting, and exotic species introductions. Spores of the coprophilous fungus Sporormiella (Fig. 1) offer the hope, from their association with animal dung, to (i) define which habitats in prehuman Madagascar supported a high biomass of large animals, (ii) yield precise estimates for the temporal association between megafaunal biomass decline and other evidence for extinction and environmental change, and (iii) provide a test of causal hypotheses for the extinctions and examine the possible role of livestock introductions (19) in these events.
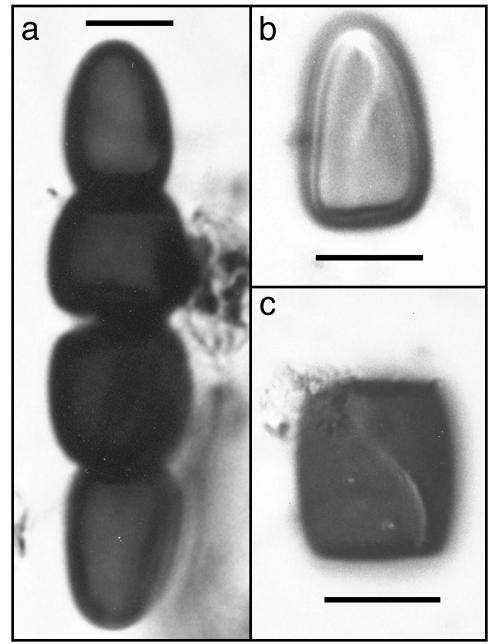
Fig. 1.
Sporormiella photomicrographs. (a) Tetrad. These are not often intact as fossils in sediments. (b) Terminal spore segment showing characteristic sigmoid aperture. (c) Note characteristic aperture in medial segment. In sediments, generic-level Sporormiella spp. identifications are readily made from pollen slides. (Scale bars, 10 μm.)
Pollen slides from sediment cores and stratigraphic excavations that we have published from throughout Madagascar (5, 7, 8, 20) were recounted for Sporormiella spp. spores following the methods described in ref. 1. Spores are expressed as percent of the basic pollen sum + Sporormiella. Age models were created by linear interpolation from the 14C dates in refs. 5, 7, 8, and 20, and additional dates supplied in Table 1. Charcoal results are from ref. 5, replotted on the new age-models, with additional levels counted to fill temporal gaps. 14C ages are reported as yr B.P. corrected for isotopic fractionation ±1σ. Dendrocalibrations (21) are expressed as range at 2σ (cal yr A.D.). These six sites sample much of the island's full range of precipitation, seasonality, elevation, and vegetation type (Fig. 2).
Table 1. Key 14C determinations used in the analysis.
| Event/taxon | Site | 14C yr B.P. | 2σ cal yr | Lab no./type | Material; treatments (source) |
|---|---|---|---|---|---|
| Spore decline | Ambolisatra | 1,720 ± 40 | A.D. 230-410 | β-167657 AMS | Plant macrofossil; acid/alkali (this paper) |
| Belo-sur-Mer* | 1,990 ± 50 | 100 B.C.-A.D. 110 | β-103348 AMS | Organic sediment; acid (5) | |
| Charcoal increase | Ambolisatra | ca. 1,500† | |||
| Belo-sur-Mer | 1,830 ± 60 | A.D. 60-350 | β-90099 AMS | Eggshell; acid-etched (5) | |
| Amparihibe | 1,130 ± 50 | A.D. 780-1010 | β-67392 AMS | Plant macrofossil; acid/alkali (5) | |
| Benavony | 710 ± 110 | A.D. 1,050-1100 and 1,140-1,430 | β-81218 Radiometric | Peat; acid/alkali; 4× normal counting time (5) | |
| Tritrivakely | 1,240 ± 100 | A.D. 630-1,000 | β-15884 Radiometric | Organic sediment; acid/alkali (7) | |
| Kavitaha | 1,400 ± 80 | A.D. 530-780 | β-14855 Radiometric | Organic sediment; acid/alkali (20) | |
| Spore increase (cattle) | Amparihibe | 1,130 ± 50 | A.D. 780-1,010 | β-67392 AMS | Same date as charcoal increase given above |
| Kavitaha | 960 ± 90 | A.D. 900-1,260 | β-15528 Radiometric | Organic sediment; acid/alkali (20) | |
| Late occurrences, extinct taxa | |||||
| Archaeolemur | Anjohikely | 830 ± 60 | A.D. 1,040-1,290 | β-55060 AMS | Fecal pellet; acid/alkali/acid (12) |
| Ankarana | 1,020 ± 50 | A.D. 910-920, 960-1060, and 1,080-1,150 | β-60797 AMS | Bone collagen; acid/alkali/acid (11) | |
| Belo-sur-Mer | 1,370 ± 40 | A.D. 620-700 | β-90095 AMS | Bone collagen; acid/alkali/acid (5) | |
| Hadropithecus | Belo-sur-Mer | 1,413 ± 80 | A.D. 444-772 | Rafter-26341/1 AMS | Bone collegen; acid (this paper) |
| Palaeopropithecus | Ankazoabo | 1,269 ± 80 | A.D. 640-964 | Rafter-10059 AMS | Tooth: acid (this paper) |
| Ankilitelo | 510 ± 80 | A.D. 1,300-1,510 and 1,600-1,620 | Not given AMS | Bone collagen: acid/alkali/acid (10) | |
| Megaladapis | Ankilitelo | 630 ± 50 | A.D. 1,280-1,420 | Not given AMS | Bone collagen: acid/alkali/acid (10) |
| Pachylemur | Belo-sur-Mer | 1,220 ± 50 | A.D. 680-910 and 920-960 | β-90098 AMS | Bone collagen; acid/alkali/acid (5) |
| Aepyornis | Belo-sur-Mer | 1,830 ± 60 | A.D. 60-350 | β-90099 AMS | Eggshell: acid-etched (5) |
| Mullerornis | Belo-sur-Mer | 1,280 ± 60 | A.D. 650-890 | β-103349 AMS | Bone collagen; acid/alkali/acid (5) |
See cited sources for other 14C dates from the sites. B.C., before Christ; AMS, accelerator mass spectrometry.
10 cm below the level of spore decline.
Estimated from age model. Sediment date from 10 cm higher, during period of heaviest charcoal influx, was 1,890 ± 90 yr B.P. (see ref. 8). Subsequent dating of a plant macrofossil from 40 cm deeper (1,720 yr B.P.) suggests this sediment date is too old by ≈600 yr, probably due to inherent age effects and/or old carbon redeposition.
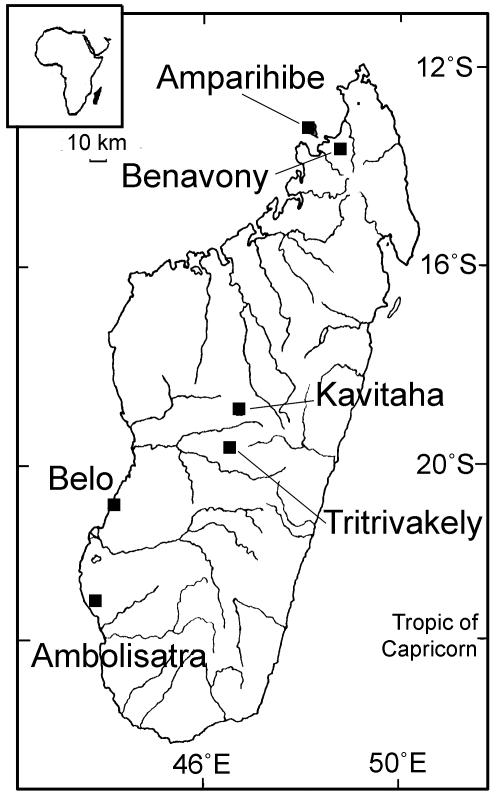
Fig. 2.
Location of sites in Madagascar. The southwestern sites are in the arid coastal strip. Ambolisatra receives ≈400 mm of annual precipitation, with high interannual variability. Belo-sur-Mer is likewise variable and receives ≈500 mm. Ambolisatra was excavated and cored at an interdunal pond site known today as Andolonomby (S23°04′/E43°45′). Excavations and coring were carried out at the Ankilibehandry site (S20°44′/E44°01′) near Belo-sur-Mer. Benavony (S13°43′/E48°29′) is a riparian marsh on the Sambirano River in the humid (2,500 mm/yr) northwest interior. A deep crater lake was cored at Lac Amparihibe (S13°18′/E48°13′) on the humid shelf island of Nosy Be (2,000 mm/yr). In the central highlands, cores were analyzed from the shallow crater lake Tritrivakely (S19°47′/E46°55′; 1,800-m elevation) and the volcanic barrier lake at Kavitaha (S19°02′/E49°34′; 1,210-m elevation).
Sporormiella values were highest in semiarid southwestern sites (Fig. 3a) before human arrival. At the coastal excavations of Ambolisatra (6, 8) and Belo-sur-Mer (5), two of the richest late Holocene “subfossil” megafaunal sites, the fungus spore maintains high values (2.3–15.1%) throughout prehuman climate changes of the late Holocene as the vegetation formations became increasingly open. However, at levels pegged to human arrival by the combination of techniques described in the introduction, the fungus spore drops to low levels and is absent in some pollen spectra over the next few centuries as charcoal values rise sharply. At Ambolisatra, where sedimentary resolution is especially good in the early part of the “extinction window,” the spore decline seems to precede the rise in charcoal by two centuries or more. Temporal resolution is not as good at Belo, but both records show ≈50 cm of organic sediment accumulation between the spore decline and the charcoal increase. At these dry sites, the spore does not increase again (signaling the presence of cattle and other introduced livestock) until well after the inferred megafaunal decline and charcoal increase. The last 1,000 years is poorly resolved in the deflated uppermost sediments of Ambolisatra and Belo, however (5, 8), and thus it is not clear from these two sites when cattle were introduced, only that Sporormiella spores are once again present in moderate percentages near the surface.
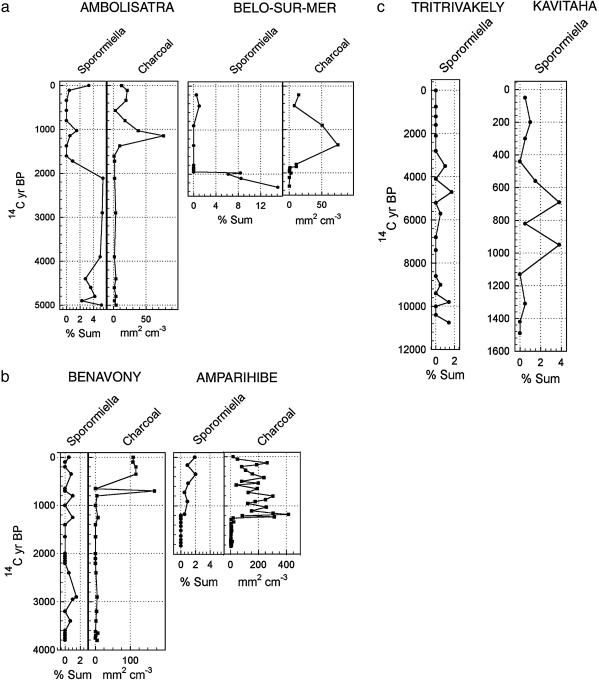
Fig. 3.
Sporormiella spore and charcoal particle analyses for six sites throughout Madagascar. Spores are expressed as percent of pollen sum + Sporormiella, and charcoal is expressed as projected area in slides per volume of sediment. (a) Coastal sites in the arid southwest show very high percentages of Sporormiella before human arrival approximately two millennia ago. Late Holocene sediments are devoid of charcoal before human arrival and for approximately two centuries after Sporormiella decline. From ≈1,720 yr B.P. to a few centuries ago, Sporormiella is absent, and charcoal reaches an initial peak and then declines. Both Sporormiella and charcoal increase in recent centuries. (b) Humid sites of the northwest, Benavony marsh on the Sambirano River and Amparihibe crater lake on the shelf island of Nosy Be, provide information on low-elevation rain-forest sites. Benavony shows low to moderate values for Sporormiella in the late Holocene before an inferred human presence. The spore is absent there and also at Amparihibe, beginning approximately two millennia ago, until 1,130 yr B.P. when a moderate Sporormiella signal may correspond to the introduction of cattle husbandry. Charcoal increases simultaneously with this spore increase at Amparihibe and several centuries later in the dense rainforest of the interior at Benavony. (c) Sporormiella results from two central highland sites show that low to moderate spore values were recorded at the Tritrivakely crater lake throughout the prehuman Holocene. At the lower and less remote site of a volcanic barrier lake at Kavitaha in the central highlands, a core spanning the last 1,500 yr shows Sporormiella increasing to moderate percentages beginning ≈960 yr B.P. Charcoal particles (data not shown; see ref. 20) increase above moderate background levels approximately four centuries earlier. See Table 1 for key 14C details and calibrations.
At Benavony marsh in the Sambirano rainforest of the northwest lowland interior (Fig. 3b), Sporormiella spores in prehuman sediments of this constantly humid, low-seasonality area are quite low (0–1.5%). The spore is absent from samples dating between ≈2,200 and 1,400 yr B.P., but occurred after the earliest attested appearance of cattle in the area approximately one millennium ago. At another humid site in the northwest, the deep crater lake Amparihibe on the shelf island of Nosy Be, a presumably livestock-derived spore increase dates to 1,130 ± 50 yr B.P. (780–1,010 cal yr A.D.) on a plant macrofossil in the decadal to annually resolved sediments. The area of these two sites, centered on Ampasindava Bay, was an early focus of settlement by Islamized Indian Ocean traders with close connections to East Africa (22, 23).
In parts of the central highlands, mesic wooded savannas occurred in the Holocene under conditions of strong seasonality and frequent prehuman fires (4). At the crater lake Tritrivakely at 1,800 m (Fig. 3c), a range of moderate to low Sporormiella values occurs in the sediments before humans. In this site, which is near the lower limit for montane ericoid bushland today, the spore is moderately frequent in the Holocene whenever the pollen record showed predominance of wooded savanna (to 1.7%) and is lower or absent when pollen of trees or ericoid shrubs show a large increase (7).
A record of the last 1,500 yr at Kavitaha, a volcanic barrier lake at 1,210-m elevation near Madagascar's well known highland fossil site, Ampasambazimba (3), provides a detailed picture of human transformation of the uplands (20). Humans indirectly show themselves to have been present locally from ≈1,400 yr B.P. (530–780 cal yr A.D.) onward, with a rise in microscopic charcoal particles and a decline in the woody component of the pollen spectra. The spores of Sporormiella become numerous (3.8%) at ≈960 yr B.P. (900–1,210 cal yr A.D.), probably signaling livestock arrival. Archaeological sites in the region confirm that human populations were present in the central highlands at least by 800 yr B.P. (24). Although it is not known for certain when pastoralism became prevalent in Madagascar, the Islamized traders of the western Indian Ocean, who had probably settled in the northwest of the island by the beginning of the second millennium A.D., could have brought cattle to Madagascar (22).
Megafaunal Paleoenvironments
The scarcity of the dung fungus spore throughout the mid- to late Holocene at Tritrivakely and Benavony suggests that megafaunal biomass in the high-elevation ericoid bushlands and humid lowland forests was relatively lower than in wooded grasslands of the semiarid southwest and central highlands. Although it is clear that many if not all of the giant lemurs were forest-adapted (25), hippos, tortoises, and elephant birds probably were not. These latter are generally more common in most fossil sites of the island than the primates (except in some cave deposits, which probably show a taphonomic bias toward climbers) (11). Nonprimate components of the megafauna almost certainly were well adapted to open country based on the fossils' coeval pollen data (6, 8) and inferences from comparison to their living relatives elsewhere in the world (4).
Sporormiella results may indicate overall that megafaunal biomass in late Holocene prehuman Madagascar was concentrated in formations that were widespread then but relatively rare today: various types of wooded savanna including semiarid southwest sites currently in the annual range of 500–800 mm of precipitation (5) up to perhaps 1,800 mm in the central highlands in areas with a long dry season. Conversely, megafaunal biomass may have been relatively low in mountain areas dominated by ericoid heath (≈2,000+ m above sea level today but covering much of the interior down to ≈1,000 m at the Last Glacial Maximum) (16). Similarly, lowland humid rainforest shows only a trace of Sporormiella before humans. Although it is possible that taphonomic processes could explain some of the observed variability, we believe it is more likely that spore frequencies indicate differences in the local density of herbivores.
In both these environments, the low spore counts cannot be explained away easily as a taphonomic artifact such as poor preservation of dung, unsuitability to the fungus, or lack of spore transport to the sediments. If these negative results were purely an artifact of palynological representation, one would expect that the introduction of livestock into these wet areas might also be poorly represented, but that is not the case. The spore shows some late Holocene increase after cattle introduction in both mesic upland and humid lowland sites.
It is reasonable to assume that most of the primate component of the megafauna lived in or near forest, because giant lemur skeletons show a broad range of arboreal adaptations. Giant lemurs such as Megaladapis spp. and Palaeopropithecus spp. were almost certainly awkward on the ground (25). On the other hand, the “monkey lemurs,” baboon-sized extinct forms such as the Archaeolemuridae, possessed features well adapted to life in the trees but also could run on the ground and feed in a wide array of habitats. Archaeolemur spp. are often more common in fossil sites than the larger extinct lemurs (25). By analogy to tropical environments elsewhere, it is likely that the productivity of closed humid forest, in terms of support for large animal biomass, was much lower than more open habitats such as mesic savannas and semiarid woodlands, where grasses, shrubs, and low trees would have provided more accessible forage and more rapid and thorough plant biomass turnover. The productivity and ecological dynamics of these more open formations are likely to have been comparable to the miombo of southern Africa or the Serengeti woodlands of East Africa, with their high megafaunal density and diversity. Humid forest and montane heath, by comparison, would be expected to sequester biomass in wood rather than cycling production rapidly through a dense assemblage of large herbivores. Also, by analogy to other tropical areas, the closed forest formations might have been expected to support a diverse primate assemblage but at relatively low density. These observations are consistent with the Sporormiella results presented here.
The ancestral Hippopotamus amphibius, a grass eater, after reaching Madagascar may have radiated into as many as four species (26). Tortoises, likewise, were quite diverse (27) and show adaptations for life in lightly wooded open country and even deserts. Ratites, from the smallest Mullerornis, not quite ostrich-sized, to the 3-m, half-ton Aepyornis maximus, were diverse, abundant, and perhaps like some other large ratites such as the ostrich, rhea, and emu, well adapted to coexistence with arid climates, open country, and predators. In the absence of very large predators except the crocodile, the big ratites, hippos, and tortoises of Madagascar might have been, as the fossil record suggests, extremely numerous, competing for scarce forage and regulated by density-dependent factors such as range quality.
Ecosystem Collapse and Extinction
With human arrival, this system seems to have collapsed rapidly, with spore-inferred biomass declining in <200 yr locally, at least in the better documented southwest sites. Whereas some large and ungainly types such as Megaladapis and Palaeopropithecus may have disappeared from all but the most inaccessible parts of the island soon after human arrival, these were still present more than one millennium later at Ankilitelo (10), a site in a remote region of the southwest that is lightly populated even today because of the rugged karst topography. Others (Table 1), such as the omnivorous Archaeolemur (12), the hippos, and perhaps the ratites, probably persisted until recent centuries even along the southwest coast (18) in areas with some of the earliest evidence for humans (5, 6).
If, probably through human overexploitation (28), megafaunal grazing and browsing pressure declined to much lower levels than normal for this vegetation, these seasonally dry areas would be likely to become much more flammable because of excess litter accumulation. This hypothetical sequence of events would explain why, in the sediments at Ambolisatra and Belo, a drastic spore decline leads charcoal increase by a few centuries. With a human presence and few large herbivores, frequent fires would probably transform more arid savannas to thorny deserts and short grasslands. Wetter savannas in the highlands could be expected to lose much of their woody element to increased fire frequency, resulting in the depauperate steppe vegetation that covers ≈70% of the land area today. Steep and rocky areas that suppress litter accumulation would form the islands of tapia woodland now scattered thinly over the highlands.
If these inferences are correct, the new pieces added to the extinction puzzle would suggest that these megafaunal extinctions might have been driven by a complex series of causal interactions including but not necessarily limited to:
The late Holocene before humans was a time of increasing aridity and perhaps seasonal or interannual dynamics that promoted changes in key megafaunal habitats such as wooded savanna.
People arrived and hunted the naive megafauna, at least the ones that were slow on the ground such as many of the largest lemurs, to local extirpation and depressed the numbers of other megafauna.
In the absence of the strong cropping regime imposed by hippos, tortoises, and ratites on the grasslands (and perhaps on woody vegetation by giant lemurs), savanna areas, forest edges, and understories would become increasingly flammable as plant biomass accumulated.
The change in fire regimes would mean that preferred megafaunal habitats became increasingly fragmented as fire converted areas to simpler systems with less edible plant biomass (e.g., spiny bushland and steppe).
Humid forest and high-elevation areas were the last settled and converted by humans (as indeed they are still being transformed today at an alarming rate) (13), but they would have provided poor refugia for most of the savanna-adapted megafauna, and the less-swift giant lemurs would have been vulnerable to hunting even within dense habitats.
Such a complex scenario is difficult to falsify directly, but it is consistent with a wide array of fossil evidence. A key role for synergistic interactions of these types in the global pattern of late Quaternary extinctions is also supported by the “Rosetta stone” (29) of historical extinctions as well as by the decline of smaller species taking place in Madagascar and the rest of the world today.
Acknowledgments
H. T. Wright, R. E. Dewar, H. James, L. Godfrey, and W. Jungers provided comments on the manuscript. This work was supported by National Science Foundation Grants BCS-0129185, BCS-0001420, DEB-9306603, and DEB 9025020, the National Geographic Society, the Smithsonian Institution, and National Oceanic and Atmospheric Administration Human Dimensions of Global Change.
Abbreviations: A.D., anno Domini; yr B.P., radiocarbon years before present (before A.D. 1950); cal yr A.D., dendrocalibrated age in calendar years A.D.
References
- 1.Davis, O. K. (1987) Quat. Res. 28, 290–294. [Google Scholar]
- 2.Davis, O. K. & Moratto, M. J. (1988) Madroño 35, 132–149. [Google Scholar]
- 3.MacPhee, R. D. E., Burney D. A. & Wells, N. A. (1985) Int. J. Primatol. 6, 463–487. [Google Scholar]
- 4.Burney, D. A. (1997) in Natural Change and Human Impact in Madagascar, eds. Goodman, S. M. & Patterson, B. D. (Smithsonian Press, Washington, DC), pp. 75–89.
- 5.Burney, D. A. (1999) in Extinctions in Near Time: Causes, Contexts, and Consequences, ed. MacPhee, R. D. E. (Plenum/Kluwer, New York), pp. 145–164.
- 6.MacPhee, R. D. E. & Burney, D. A. (1991) J. Archaeol. Sci. 18, 695–706. [Google Scholar]
- 7.Burney, D. A. (1987) Palaeoecol. Afr. 18, 357–381. [Google Scholar]
- 8.Burney, D. A. (1993) Quat. Res. 40, 98–106. [Google Scholar]
- 9.Dahl, O. (1951) Malgache et Maanyan (Egede Institutett, Oslo).
- 10.Simons, E. L. (1997) in Natural Change and Human Impact in Madagascar, eds. Goodman, S. M. & Patterson, B. D. (Smithsonian Press, Washington, DC), pp. 142–168.
- 11.Simons, E. L., Burney, D. A., Chatrath, P. S., Godfrey, L. R., Jungers, W. L. & Rakotosamimanana, B. (1995) Quat. Res. 43, 249–254. [Google Scholar]
- 12.Burney, D. A., James, H. F., Grady, F. V., Rafamantanantsoa, J.-G., Ramilisonina, Wright, H. T. & Cowart, J. B. (1997) J. Biogeogr. 24, 755–767. [Google Scholar]
- 13.Green, G. M. & Sussmann, R. W. (1990) Science 248, 212–215. [DOI] [PubMed] [Google Scholar]
- 14.Gasse, F., Cortijo, E., Disnar, J., Ferry, L., Gibert, E., Kissel, C. Laggound-Defarge, F., Lallier-Vergès, E., Miskovsky, J., Ratsimbazafy, B., et al. (1994) C. R. Acad. Sci. 318, 1513–1519. [Google Scholar]
- 15.Burney, D. A. (1988) Palaeogeogr. Palaeoclimatol. Palaeoecol. 66, 63–75. [Google Scholar]
- 16.Burney, D. A. (1996) in Biogeographie de Madagascar, ed. Lourenço, W. (ORSTOM, Paris), pp. 49–58.
- 17.Godfrey, L. R. (1986) Sciences (NY) 1986, 49–51. [Google Scholar]
- 18.Burney, D. A. & Ramilisonina. (1998) Am. Anthropol. 100, 957–966. [Google Scholar]
- 19.Dewar, R. E. (1984) in Quaternary Extinctions: A Prehistoric Revolution, eds. Martin, P. S. & Klein, R. G. (Univ. of Arizona Press, Tucson), pp. 574–593.
- 20.Burney, D. A. (1987) Quat. Res. 28, 130–143. [Google Scholar]
- 21.Stuiver, M., Reimer, P. J., Bard, E., Beck, J. W., Burr, G. S., Hughen, K. A., Kromer, B., McCormac, F. G., Plicht, J. v. d. & Spurk. M. (1998) Radiocarbon 40, 1041–1083. [Google Scholar]
- 22.Dewar, R. E. & Wright, H. T. (1993) J. World Prehist. 7, 417–466. [Google Scholar]
- 23.Radimalahy, C. (1998) Mahilaka: Archaeological Studies of an Early Port in Northwestern Madagascar. Studies in African Archaeology No. 15 (Dept. of Archaeology and Ancient History, Societas Archeologica Uppsaliensis, Uppsala).
- 24.Wright, H. T., Andrianalvoarivony, R., Bailiff, I., Burney, D., Haas, H., Raharijaona, V., Rakotovololona, S., Rasamuel, D. & Dewar, R. (1992) Taloha 11, 121–146. [Google Scholar]
- 25.Godfrey, L. R. & Jungers, W. L. (2002) in The Primate Fossil Record, ed. Hartwig, W. (Cambridge Univ. Press, Cambridge, U.K.), pp. 97–122.
- 26.Steunes, S. (1989) J. Vert. Paleontol. 9, 241–268. [Google Scholar]
- 27.Palkovacs, E. P., Gerlach, J. & Caccone, A. (2002) Mol. Phylogenet. Evol. 24, 216–227. [DOI] [PubMed] [Google Scholar]
- 28.Martin, P. S. & Steadman, D. W. (1999) in Extinctions in Near Time: Causes, Contexts, and Consequences, ed. MacPhee, R. D. E. (Plenum/Kluwer, New York), pp. 17–55.
- 29.Diamond, J. M. (1984) in Quaternary Extinctions: A Prehistoric Revolution, eds. Martin, P. S. & Klein, R. G. (Univ. of Arizona Press, Tucson), pp. 824–862.