Abstract
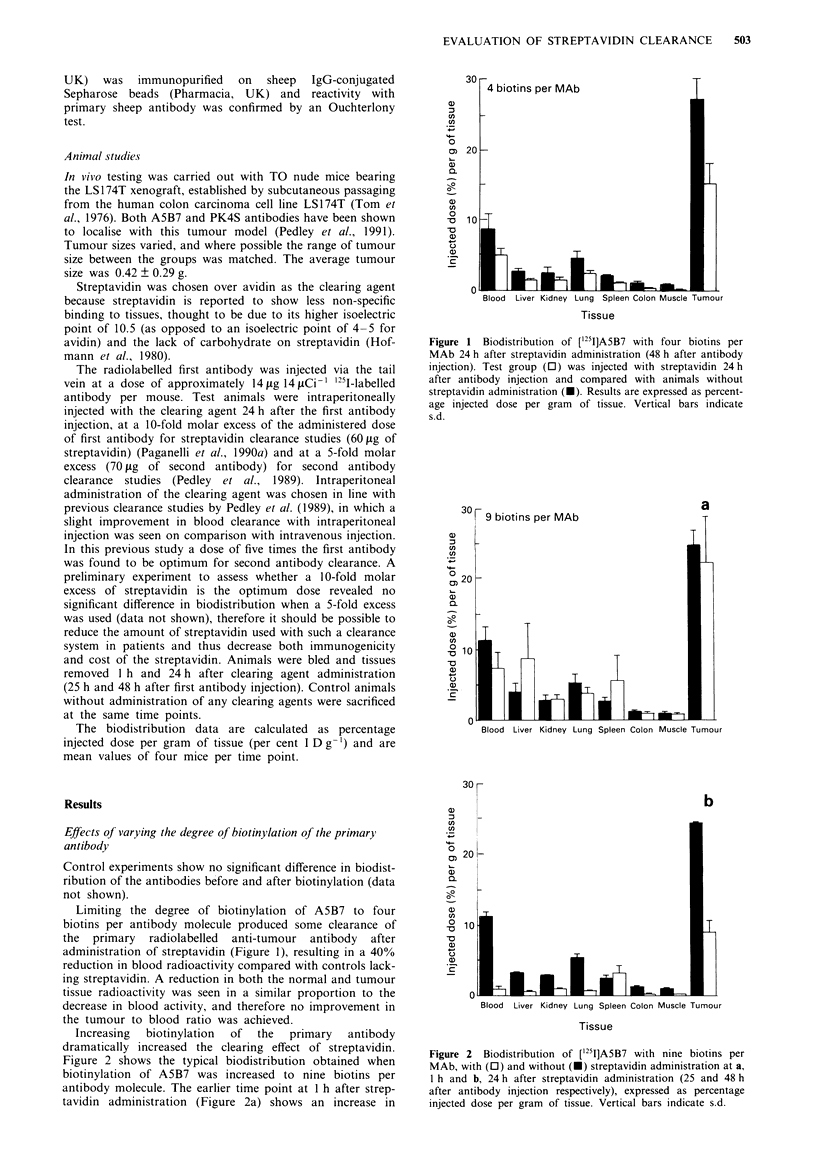
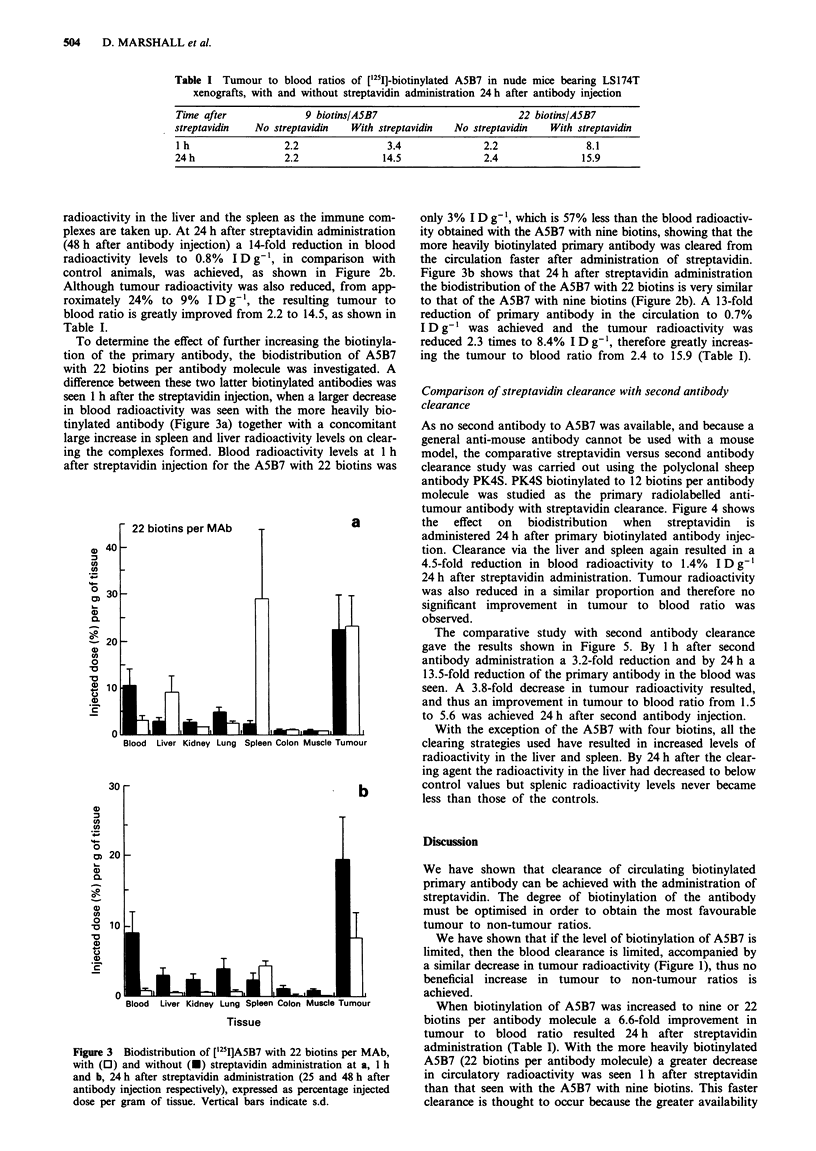
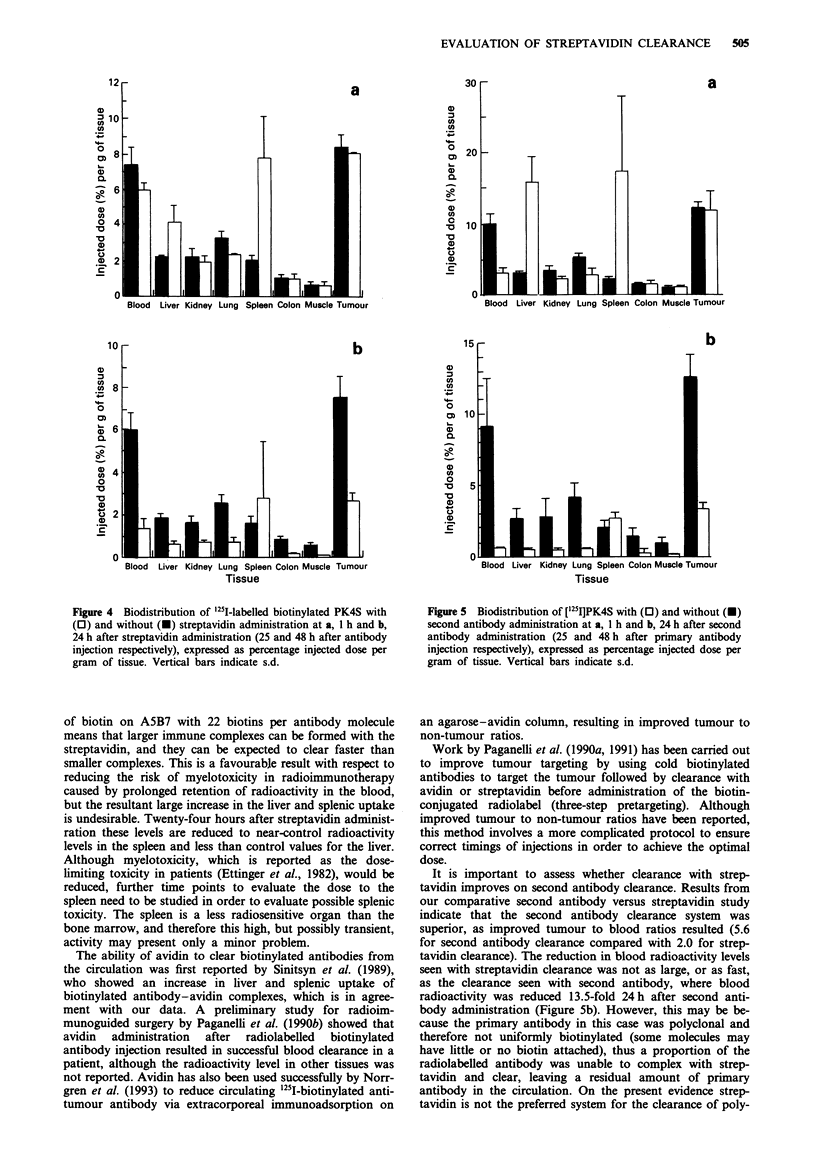
The improved tumour to non-tumour ratios needed for effective tumour targeting with antibodies requires that blood background radioactivity is reduced. We investigated the effect of streptavidin as a clearing agent for 125I-labelled biotinylated anti-CEA antibodies in a human colon carcinoma xenograft model. By comparing the biodistribution of the monoclonal antibody A5B7 with four, nine or 22 biotins per antibody molecule, we investigated how the degree of biotinylation of the primary radiolabelled antibody affects its clearance with streptavidin. Limiting the degree of biotinylation limited blood clearance, whereas nine or 22 biotins per antibody molecule resulted in a 13- to 14-fold reduction in blood radioactivity, the streptavidin-biotinylated antibody complexes clearing rapidly via the liver and spleen. Although a reduction in tumour activity was also seen, a 6.6-fold improvement in the tumour to blood ratio was achieved. A comparative study of streptavidin versus second antibody clearance was carried out using the polyclonal antibody PK4S biotinylated with 12 biotins per antibody molecule. This study indicated that second antibody was superior for clearance of the polyclonal antibody, resulting in a larger and faster reduction in blood radioactivity and improved tumour to blood ratios. In this case the primary antibody was polyclonal, and therefore non-uniformity of biotinylation may affect complexation with streptavidin. Therefore, the degree of biotinylation and type of antibody must be carefully considered before the use of streptavidin clearance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Wilchek M. Protein biotinylation. Methods Enzymol. 1990;184:138–160. doi: 10.1016/0076-6879(90)84268-l. [DOI] [PubMed] [Google Scholar]
- Begent R. H., Bagshawe K. D., Pedley R. B., Searle F., Ledermann J. A., Green A. J., Keep P. A., Chester K. A., Glaser M. G., Dale R. G. Use of second antibody in radioimmunotherapy. NCI Monogr. 1987;(3):59–61. [PubMed] [Google Scholar]
- Begent R. H., Keep P. A., Green A. J., Searle F., Bagshawe K. D., Jewkes R. F., Jones B. E., Barratt G. M., Ryman B. E. Liposomally entrapped second antibody improves tumour imaging with radiolabelled (first) antitumour antibody. Lancet. 1982 Oct 2;2(8301):739–742. doi: 10.1016/s0140-6736(82)90923-0. [DOI] [PubMed] [Google Scholar]
- Blumenthal R. D., Sharkey R. M., Snyder D., Goldenberg D. M. Reduction by anti-antibody administration of the radiotoxicity associated with 131I-labeled antibody to carcinoembryonic antigen in cancer radioimmunotherapy. J Natl Cancer Inst. 1989 Feb 1;81(3):194–199. doi: 10.1093/jnci/81.3.194. [DOI] [PubMed] [Google Scholar]
- Ettinger D. S., Order S. E., Wharam M. D., Parker M. K., Klein J. L., Leichner P. K. Phase I-II study of isotopic immunoglobulin therapy for primary liver cancer. Cancer Treat Rep. 1982 Feb;66(2):289–297. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GREEN N. M. A SPECTROPHOTOMETRIC ASSAY FOR AVIDIN AND BIOTIN BASED ON BINDING OF DYES BY AVIDIN. Biochem J. 1965 Mar;94:23C–24C. doi: 10.1042/bj0940023c. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. M., Sharkey R. M., Ford E. Anti-antibody enhancement of iodine-131 anti-CEA radioimmunodetection in experimental and clinical studies. J Nucl Med. 1987 Oct;28(10):1604–1610. [PubMed] [Google Scholar]
- Hofmann K., Wood S. W., Brinton C. C., Montibeller J. A., Finn F. M. Iminobiotin affinity columns and their application to retrieval of streptavidin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4666–4668. doi: 10.1073/pnas.77.8.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalofonos H. P., Rusckowski M., Siebecker D. A., Sivolapenko G. B., Snook D., Lavender J. P., Epenetos A. A., Hnatowich D. J. Imaging of tumor in patients with indium-111-labeled biotin and streptavidin-conjugated antibodies: preliminary communication. J Nucl Med. 1990 Nov;31(11):1791–1796. [PubMed] [Google Scholar]
- Keep P. A., Searle F., Begent R. H., Barratt G. M., Boden J., Bagshawe K. D., Ryman B. E. Clearance of injected radioactively labelled antibodies to tumour products by liposome-bound second antibodies. Oncodev Biol Med. 1983;4(4):273–280. [PubMed] [Google Scholar]
- Ledermann J. A., Begent R. H., Bagshawe K. D., Riggs S. J., Searle F., Glaser M. G., Green A. J., Dale R. G. Repeated antitumour antibody therapy in man with suppression of the host response by cyclosporin A. Br J Cancer. 1988 Nov;58(5):654–657. doi: 10.1038/bjc.1988.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrgren K., Strand S. E., Nilsson R., Lindgren L., Sjögren H. O. A general, extracorporeal immunoadsorption method to increase the tumor-to-normal tissue ratio in radioimmunoimaging and radioimmunotherapy. J Nucl Med. 1993 Mar;34(3):448–454. [PubMed] [Google Scholar]
- Paganelli G., Belloni C., Magnani P., Zito F., Pasini A., Sassi I., Meroni M., Mariani M., Vignali M., Siccardi A. G. Two-step tumour targetting in ovarian cancer patients using biotinylated monoclonal antibodies and radioactive streptavidin. Eur J Nucl Med. 1992;19(5):322–329. doi: 10.1007/BF00177053. [DOI] [PubMed] [Google Scholar]
- Paganelli G., Magnani P., Zito F., Villa E., Sudati F., Lopalco L., Rossetti C., Malcovati M., Chiolerio F., Seccamani E. Three-step monoclonal antibody tumor targeting in carcinoembryonic antigen-positive patients. Cancer Res. 1991 Nov 1;51(21):5960–5966. [PubMed] [Google Scholar]
- Paganelli G., Pervez S., Siccardi A. G., Rowlinson G., Deleide G., Chiolerio F., Malcovati M., Scassellati G. A., Epenetos A. A. Intraperitoneal radio-localization of tumors pre-targeted by biotinylated monoclonal antibodies. Int J Cancer. 1990 Jun 15;45(6):1184–1189. doi: 10.1002/ijc.2910450632. [DOI] [PubMed] [Google Scholar]
- Paganelli G., Stella M., De Nardi P., Magnani P., Zito F., Siccardi A. G., Di Carlo V., Fazio F. A new method for faster blood clearance in radioimmuno-guided surgery. J Nucl Biol Med. 1991 Apr-Jun;35(2):88–89. [PubMed] [Google Scholar]
- Pedley R. B., Begent R. H., Boden J. A., Boden R., Adam T., Bagshawe K. D. The effect of radiosensitizers on radio-immunotherapy, using 131I-labelled anti-CEA antibodies in a human colonic xenograft model. Int J Cancer. 1991 Feb 20;47(4):597–602. doi: 10.1002/ijc.2910470420. [DOI] [PubMed] [Google Scholar]
- Pedley R. B., Boden J., Keep P. A., Harwood P. J., Green A. J., Rogers G. T. Relationship between tumour size and uptake of radiolabelled anti-CEA in a colon tumour xenograft. Eur J Nucl Med. 1987;13(4):197–202. doi: 10.1007/BF00256491. [DOI] [PubMed] [Google Scholar]
- Pedley R. B., Dale R., Boden J. A., Begent R. H., Keep P. A., Green A. J. The effect of second antibody clearance on the distribution and dosimetry of radiolabelled anti-CEA antibody in a human colonic tumor xenograft model. Int J Cancer. 1989 Apr 15;43(4):713–718. doi: 10.1002/ijc.2910430429. [DOI] [PubMed] [Google Scholar]
- Sharkey R. M., Mabus J., Goldenberg D. M. Factors influencing anti-antibody enhancement of tumor targeting with antibodies in hamsters with human colonic tumor xenografts. Cancer Res. 1988 Apr 15;48(8):2005–2009. [PubMed] [Google Scholar]
- Sharkey R. M., Primus F. J., Goldenberg D. M. Second antibody clearance of radiolabeled antibody in cancer radioimmunodetection. Proc Natl Acad Sci U S A. 1984 May;81(9):2843–2846. doi: 10.1073/pnas.81.9.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinitsyn V. V., Mamontova A. G., Checkneva Y. Y., Shnyra A. A., Domogatsky S. P. Rapid blood clearance of biotinylated IgG after infusion of avidin. J Nucl Med. 1989 Jan;30(1):66–69. [PubMed] [Google Scholar]
- Tom B. H., Rutzky L. P., Jakstys M. M., Oyasu R., Kaye C. I., Kahan B. D. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976 Mar;12(3):180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]


