Abstract
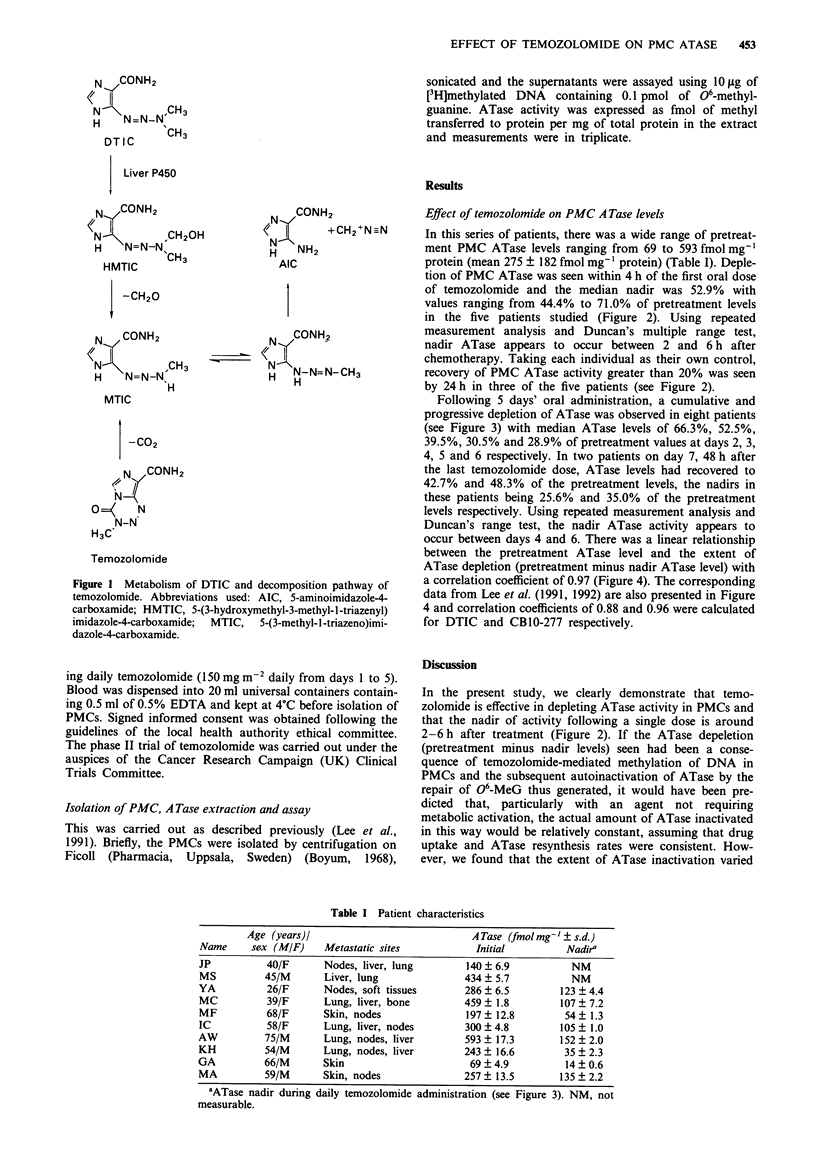
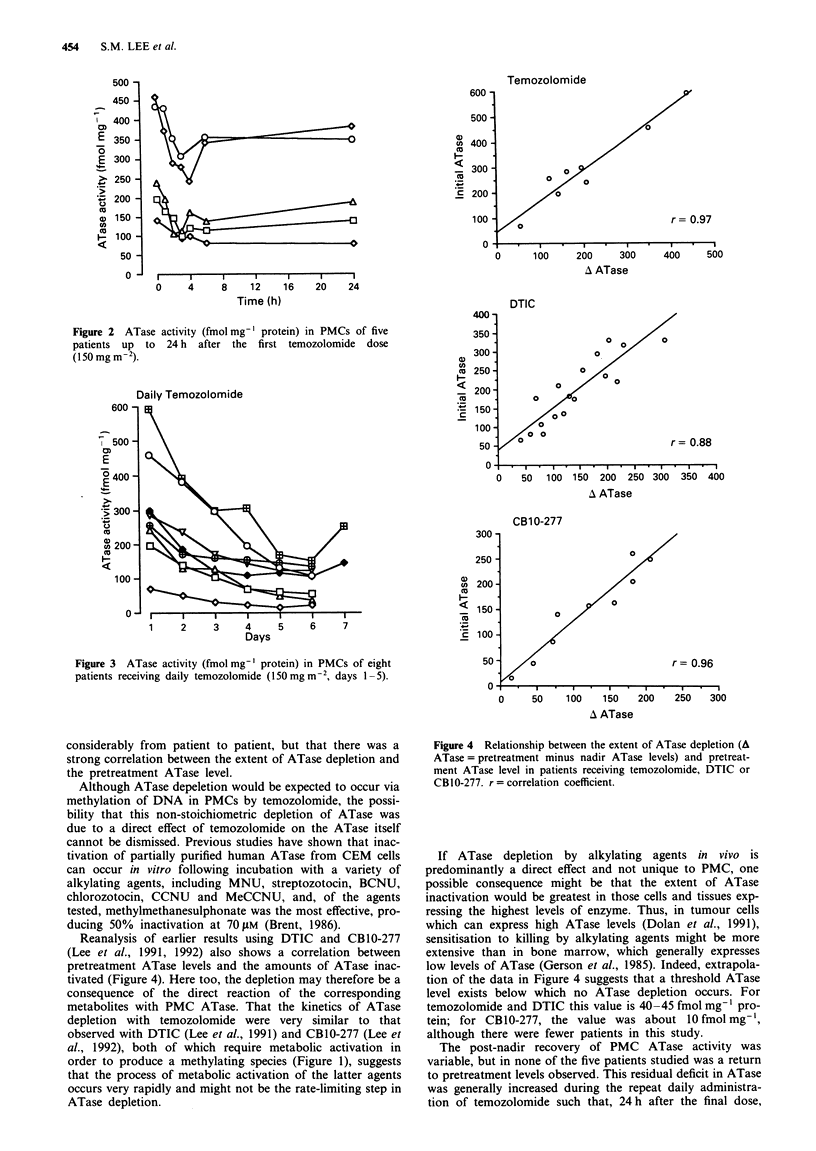
O6-alkylguanine-DNA-alkyltransferase (ATase) activity was measured in extracts of peripheral blood mononuclear cells (PMCs) taken from eight patients at various times during 5 days of oral treatment with temozolomide (150 mg m-2, days 1-5). Pretreatment ATase levels ranged from approximately 70 to 600 fmol per mg of protein. Depletion of PMC ATase was seen within 4 h of the first dose of temozolomide and had a median nadir of 52.9% and values ranging from 44.4% to 71.0% of pretreatment levels. There was a correlation between the extent of ATase depletion (pretreatment minus nadir level) and the pretreatment ATase level (r = 0.97). A progressive depletion of ATase was observed during the 5 days of continuous temozolomide therapy with median ATase activities of 66.3%, 52.5%, 39.5%, 30.5% and 28.9% of the pretreatment values at days 2, 3, 4, 5 and 6 respectively. This suggests that the schedule-dependent anti-tumour activity of temozolomide seen in experimental models and clinics may be related to a cumulative depletion of ATase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida T., Cheitlin R. A., Bodell W. J. Inhibition of O6-alkylguanine-DNA-alkyltransferase activity potentiates cytotoxicity and induction of SCEs in human glioma cells resistant to 1,3-bis(2-chloroethyl)-1-nitrosourea. Carcinogenesis. 1987 Sep;8(9):1219–1223. doi: 10.1093/carcin/8.9.1219. [DOI] [PubMed] [Google Scholar]
- Baer J. C., Freeman A. A., Newlands E. S., Watson A. J., Rafferty J. A., Margison G. P. Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br J Cancer. 1993 Jun;67(6):1299–1302. doi: 10.1038/bjc.1993.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand J., Margison G. P. Reduction of the toxicity and mutagenicity of alkylating agents in mammalian cells harboring the Escherichia coli alkyltransferase gene. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6292–6296. doi: 10.1073/pnas.83.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P. Inactivation of purified human O6-alkylguanine-DNA alkyltransferase by alkylating agents or alkylated DNA. Cancer Res. 1986 May;46(5):2320–2323. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- D'Incalci M., Citti L., Taverna P., Catapano C. V. Importance of the DNA repair enzyme O6-alkyl guanine alkyltransferase (AT) in cancer chemotherapy. Cancer Treat Rev. 1988 Dec;15(4):279–292. doi: 10.1016/0305-7372(88)90026-6. [DOI] [PubMed] [Google Scholar]
- Dempke W., Nehls P., Wandl U., Soll D., Schmidt C. G., Osieka R. Increased cytotoxicity of 1-(2-chloroethyl)-1-nitroso-3(4-methyl)-cyclohexylurea by pretreatment with O6-methylguanine in resistant but not in sensitive human melanoma cells. J Cancer Res Clin Oncol. 1987;113(4):387–391. doi: 10.1007/BF00397725. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Mitchell R. B., Mummert C., Moschel R. C., Pegg A. E. Effect of O6-benzylguanine analogues on sensitivity of human tumor cells to the cytotoxic effects of alkylating agents. Cancer Res. 1991 Jul 1;51(13):3367–3372. [PubMed] [Google Scholar]
- Gerson S. L., Miller K., Berger N. A. O6 alkylguanine-DNA alkyltransferase activity in human myeloid cells. J Clin Invest. 1985 Dec;76(6):2106–2114. doi: 10.1172/JCI112215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson S. L., Trey J. E., Miller K. Potentiation of nitrosourea cytotoxicity in human leukemic cells by inactivation of O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1988 Mar 15;48(6):1521–1527. [PubMed] [Google Scholar]
- Gibson N. W., Hartley J. A., Barnes D., Erickson L. C. Combined effects of streptozotocin and mitozolomide against four human cell lines of the Mer+ phenotype. Cancer Res. 1986 Oct;46(10):4995–4998. [PubMed] [Google Scholar]
- Gonzaga P. E., Potter P. M., Niu T. Q., Yu D., Ludlum D. B., Rafferty J. A., Margison G. P., Brent T. P. Identification of the cross-link between human O6-methylguanine-DNA methyltransferase and chloroethylnitrosourea-treated DNA. Cancer Res. 1992 Nov 1;52(21):6052–6058. [PubMed] [Google Scholar]
- Jelinek J., Kleibl K., Dexter T. M., Margison G. P. Transfection of murine multi-potent haemopoietic stem cells with an E. coli DNA alkyltransferase gene confers resistance to the toxic effects of alkylating agents. Carcinogenesis. 1988 Jan;9(1):81–87. doi: 10.1093/carcin/9.1.81. [DOI] [PubMed] [Google Scholar]
- Kaina B., Fritz G., Mitra S., Coquerelle T. Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: the role of MGMT in protection against the genotoxic effects of alkylating agents. Carcinogenesis. 1991 Oct;12(10):1857–1867. doi: 10.1093/carcin/12.10.1857. [DOI] [PubMed] [Google Scholar]
- Karran P., Williams S. A. The cytotoxic and mutagenic effects of alkylating agents on human lymphoid cells are caused by different DNA lesions. Carcinogenesis. 1985 May;6(5):789–792. doi: 10.1093/carcin/6.5.789. [DOI] [PubMed] [Google Scholar]
- Kataoka H., Hall J., Karran P. Complementation of sensitivity to alkylating agents in Escherichia coli and Chinese hamster ovary cells by expression of a cloned bacterial DNA repair gene. EMBO J. 1986 Dec 1;5(12):3195–3200. doi: 10.1002/j.1460-2075.1986.tb04629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Margison G. P., Woodcock A. A., Thatcher N. Sequential administration of varying doses of dacarbazine and fotemustine in advanced malignant melanoma. Br J Cancer. 1993 Jun;67(6):1356–1360. doi: 10.1038/bjc.1993.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Thatcher N., Crowther D., Margison G. P. In vivo depletion of O6-alkylguanine-DNA-alkyltransferase in lymphocytes and melanoma of patients treated with CB 10-277, a new DTIC analogue. Cancer Chemother Pharmacol. 1992;31(3):240–246. doi: 10.1007/BF00685554. [DOI] [PubMed] [Google Scholar]
- Lee S. M., Thatcher N., Dougal M., Margison G. P. Dosage and cycle effects of dacarbazine (DTIC) and fotemustine on O6-alkylguanine-DNA alkyltransferase in human peripheral blood mononuclear cells. Br J Cancer. 1993 Feb;67(2):216–221. doi: 10.1038/bjc.1993.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Thatcher N., Margison G. P. O6-alkylguanine-DNA alkyltransferase depletion and regeneration in human peripheral lymphocytes following dacarbazine and fotemustine. Cancer Res. 1991 Jan 15;51(2):619–623. [PubMed] [Google Scholar]
- Newlands E. S., Blackledge G. R., Slack J. A., Rustin G. J., Smith D. B., Stuart N. S., Quarterman C. P., Hoffman R., Stevens M. F., Brampton M. H. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer. 1992 Feb;65(2):287–291. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly S. M., Newlands E. S., Glaser M. G., Brampton M., Rice-Edwards J. M., Illingworth R. D., Richards P. G., Kennard C., Colquhoun I. R., Lewis P. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer. 1993;29A(7):940–942. doi: 10.1016/s0959-8049(05)80198-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Byers T. L. Repair of DNA containing O6-alkylguanine. FASEB J. 1992 Mar;6(6):2302–2310. doi: 10.1096/fasebj.6.6.1544541. [DOI] [PubMed] [Google Scholar]
- Samson L., Derfler B., Waldstein E. A. Suppression of human DNA alkylation-repair defects by Escherichia coli DNA-repair genes. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5607–5610. doi: 10.1073/pnas.83.15.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. F., Hickman J. A., Langdon S. P., Chubb D., Vickers L., Stone R., Baig G., Goddard C., Gibson N. W., Slack J. A. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987 Nov 15;47(22):5846–5852. [PubMed] [Google Scholar]
- Tong W. P., Kirk M. C., Ludlum D. B. Formation of the cross-link 1-[N3-deoxycytidyl),2-[N1-deoxyguanosinyl]ethane in DNA treated with N,N'-bis(2-chloroethyl)-N-nitrosourea. Cancer Res. 1982 Aug;42(8):3102–3105. [PubMed] [Google Scholar]
- Tsang L. L., Quarterman C. P., Gescher A., Slack J. A. Comparison of the cytotoxicity in vitro of temozolomide and dacarbazine, prodrugs of 3-methyl-(triazen-1-yl)imidazole-4-carboxamide. Cancer Chemother Pharmacol. 1991;27(5):342–346. doi: 10.1007/BF00688855. [DOI] [PubMed] [Google Scholar]
- Zlotogorski C., Erickson L. C. Pretreatment of human colon tumor cells with DNA methylating agents inhibits their ability to repair chloroethyl monoadducts. Carcinogenesis. 1984 Jan;5(1):83–87. doi: 10.1093/carcin/5.1.83. [DOI] [PubMed] [Google Scholar]
- Zlotogorski C., Erickson L. C. Pretreatment of normal human fibroblasts and human colon carcinoma cells with MNNG allows chloroethylnitrosourea to produce DNA interstrand crosslinks not observed in cells treated with chloroethylnitrosourea alone. Carcinogenesis. 1983;4(6):759–763. doi: 10.1093/carcin/4.6.759. [DOI] [PubMed] [Google Scholar]


