Abstract
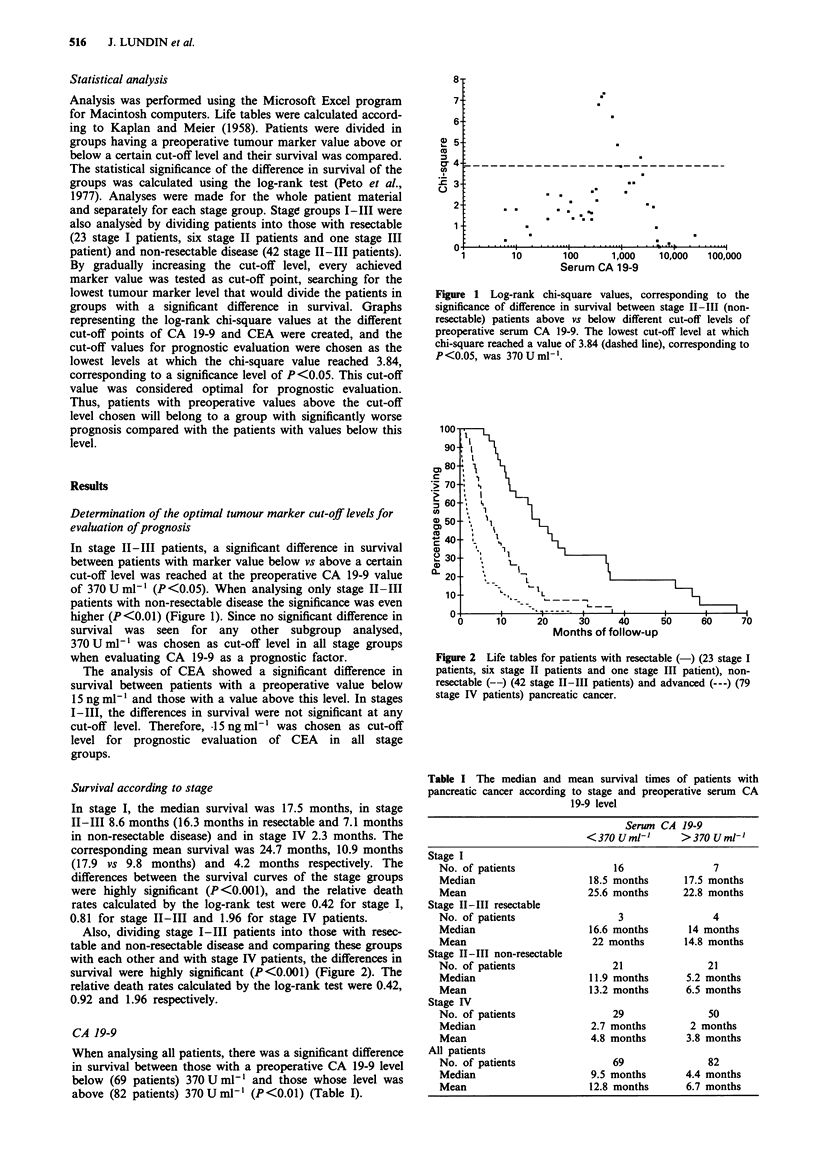
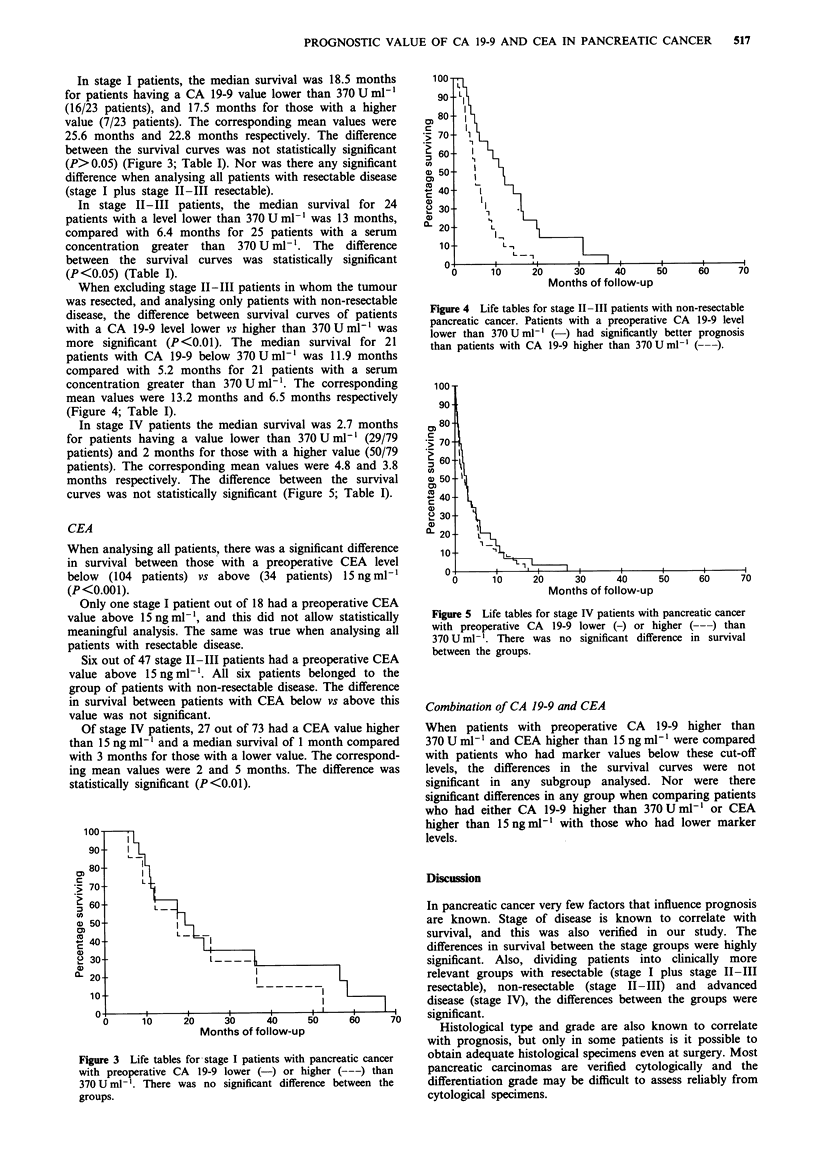
The prognostic value of preoperative serum levels of CA 19-9 and CEA was evaluated in 160 patients with pancreatic cancer. The survival of patients whose tumour marker value was below a certain cut-off level was compared with the survival of those with a higher value using the log-rank test. The lowest cut-off level dividing patients into groups with significant difference in survival (P < 0.05) was determined by graphical analysis of chi-square values at different cut-off levels. If stage of disease was not taken into account, there was a significant difference in survival between patients with low vs high preoperative CA 19-9 and CEA levels. When patients were classified according to stage, a difference was found for CA 19-9 in stage II-III patients. Patients with preoperative CA 19-9 below 370 U ml-1 had a significantly better prognosis than those with a higher level (P < 0.05). In stage I and stage IV patients, no significant difference was found between the groups at any cut-off level. The analysis of CEA showed a significant difference in survival only in stage IV patients, with CEA above 15 ng ml-1 being associated with shorter survival. In conclusion, in patients with stage II-III disease, particularly in patients with a non-resectable tumour, in whom the exact spread of the disease may be difficult to evaluate even at operation, the preoperative CA 19-9 level seems to have a prognostic value.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrén-Sandberg A., Ihse I. Factors influencing survival after total pancreatectomy in patients with pancreatic cancer. Ann Surg. 1983 Nov;198(5):605–610. doi: 10.1097/00000658-198311000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkin J. S., Kalser M. H., Kaplan R., Redlhammer D., Heal A. Initial levels of CEA and their rate of change in pancreatic carcinoma following surgery chemotherapy and radiation therapy. Cancer. 1978 Sep;42(3 Suppl):1472–1476. doi: 10.1002/1097-0142(197809)42:3+<1472::aid-cncr2820420817>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Barone D., Onetto M., Conio M., Paganuzzi M., Saccomanno S., Aste H., Pugliese V. CA 19-9 assay in patients with extrahepatic cholestatic jaundice. Int J Biol Markers. 1988 Apr-Jun;3(2):95–100. doi: 10.1177/172460088800300204. [DOI] [PubMed] [Google Scholar]
- Benini L., Cavallini G., Zordan D., Rizzotti P., Rigo L., Brocco G., Perobelli L., Zanchetta M., Pederzoli P., Scuro L. A. A clinical evaluation of monoclonal (CA19-9, CA50, CA12-5) and polyclonal (CEA, TPA) antibody-defined antigens for the diagnosis of pancreatic cancer. Pancreas. 1988;3(1):61–66. doi: 10.1097/00006676-198802000-00011. [DOI] [PubMed] [Google Scholar]
- Böttger T., Zech J., Weber W., Sorger K., Junginger T. Relevant factors in the prognosis of ductal pancreatic carcinoma. Acta Chir Scand. 1990 Nov-Dec;156(11-12):781–788. [PubMed] [Google Scholar]
- Cameron J. L., Crist D. W., Sitzmann J. V., Hruban R. H., Boitnott J. K., Seidler A. J., Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991 Jan;161(1):120–125. doi: 10.1016/0002-9610(91)90371-j. [DOI] [PubMed] [Google Scholar]
- Carr-Locke D. L. Serum and pancreatic juice carcinoembryonic antigen in pancreatic and biliary disease. Gut. 1980 Aug;21(8):656–661. doi: 10.1136/gut.21.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD P., FREEDMAN S. O. DEMONSTRATION OF TUMOR-SPECIFIC ANTIGENS IN HUMAN COLONIC CARCINOMATA BY IMMUNOLOGICAL TOLERANCE AND ABSORPTION TECHNIQUES. J Exp Med. 1965 Mar 1;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson B. Cancer of the pancreas. 50 years of surgery. Cancer. 1987 Nov 1;60(9):2284–2303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Haglund C., Kuusela P., Roberts P. J. Tumour markers in pancreatic cancer. Ann Chir Gynaecol. 1989;78(1):41–53. [PubMed] [Google Scholar]
- Haglund C., Roberts P. J., Kuusela P., Scheinin T. M., Mäkelä O., Jalanko H. Evaluation of CA 19-9 as a serum tumour marker in pancreatic cancer. Br J Cancer. 1986 Feb;53(2):197–202. doi: 10.1038/bjc.1986.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalser M. H., Barkin J. S., Redlhammer D., Heal A. Circulating carcinoembryonic antigen in pancreatic carcinoma. Cancer. 1978 Sep;42(3 Suppl):1468–1471. doi: 10.1002/1097-0142(197809)42:3+<1468::aid-cncr2820420816>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Klapdor R., Klapdor U., Bahlo M., Dallek M., Kremer B., van Ackeren H., Schreiber H. W., Greten H. CA 12-5 bei Karzinomen des Verdauungstraktes. Ein Vergleich mit CA 19-9 und CEA bei Karzinomen des Pankreas und Kolon. Dtsch Med Wochenschr. 1984 Dec 21;109(51-52):1949–1954. doi: 10.1055/s-2008-1069483. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Brockhaus M., Blaszczyk M., Magnani J., Steplewski Z., Ginsburg V. Lewis blood-type may affect the incidence of gastrointestinal cancer. Lancet. 1982 Jun 12;1(8285):1332–1333. doi: 10.1016/s0140-6736(82)92402-3. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Masson P., Pålsson B., Andrén-Sandberg A. Cancer-associated tumour markers CA 19-9 and CA-50 in patients with pancreatic cancer with special reference to the Lewis blood cell status. Br J Cancer. 1990 Jul;62(1):118–121. doi: 10.1038/bjc.1990.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganuzzi M., Onetto M., Marroni P., Barone D., Conio M., Aste H., Pugliese V. CA 19-9 and CA 50 in benign and malignant pancreatic and biliary diseases. Cancer. 1988 May 15;61(10):2100–2108. doi: 10.1002/1097-0142(19880515)61:10<2100::aid-cncr2820611028>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W. M., Gelfand R., Anderson K. K., Glenn J., Kurtzman S. H., Sindelar W. F., Toskes P. P. Comparison of the sensitivity and specificity of the CA19-9 and carcinoembryonic antigen assays in detecting cancer of the pancreas. Gastroenterology. 1986 Feb;90(2):343–349. doi: 10.1016/0016-5085(86)90930-3. [DOI] [PubMed] [Google Scholar]
- Trede M., Schwall G., Saeger H. D. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990 Apr;211(4):447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]


