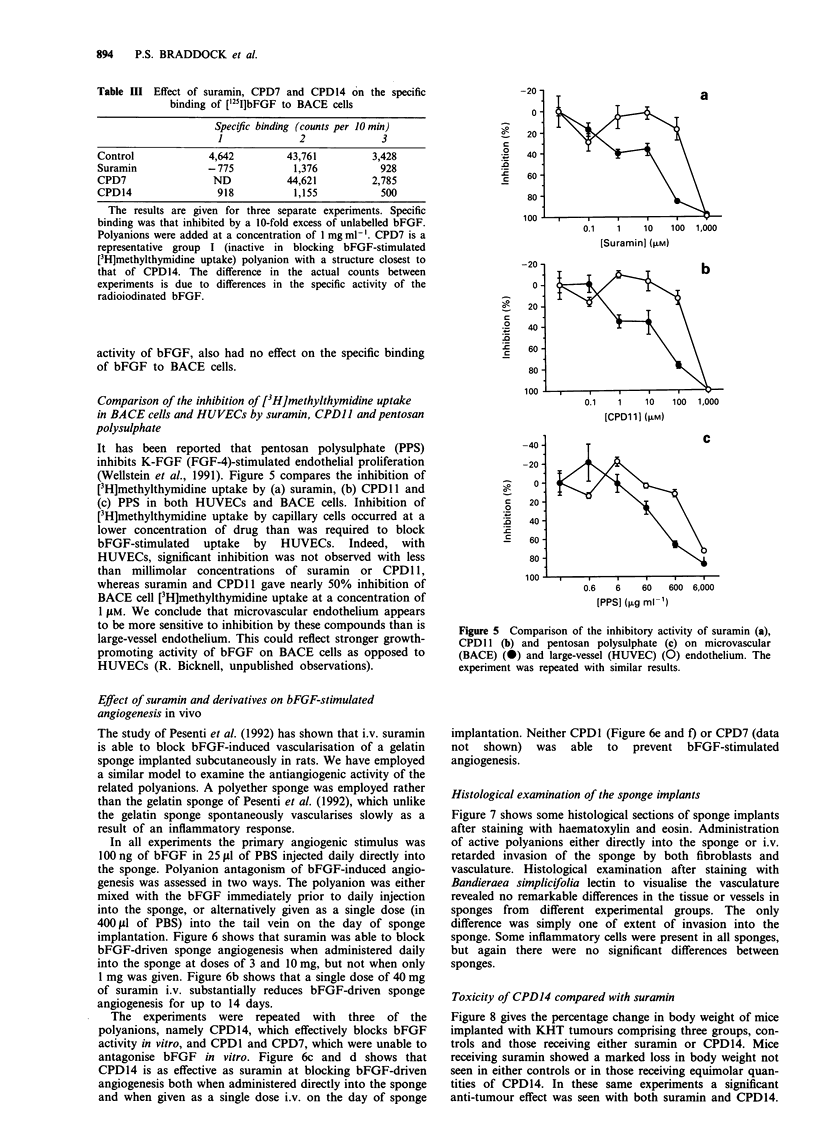
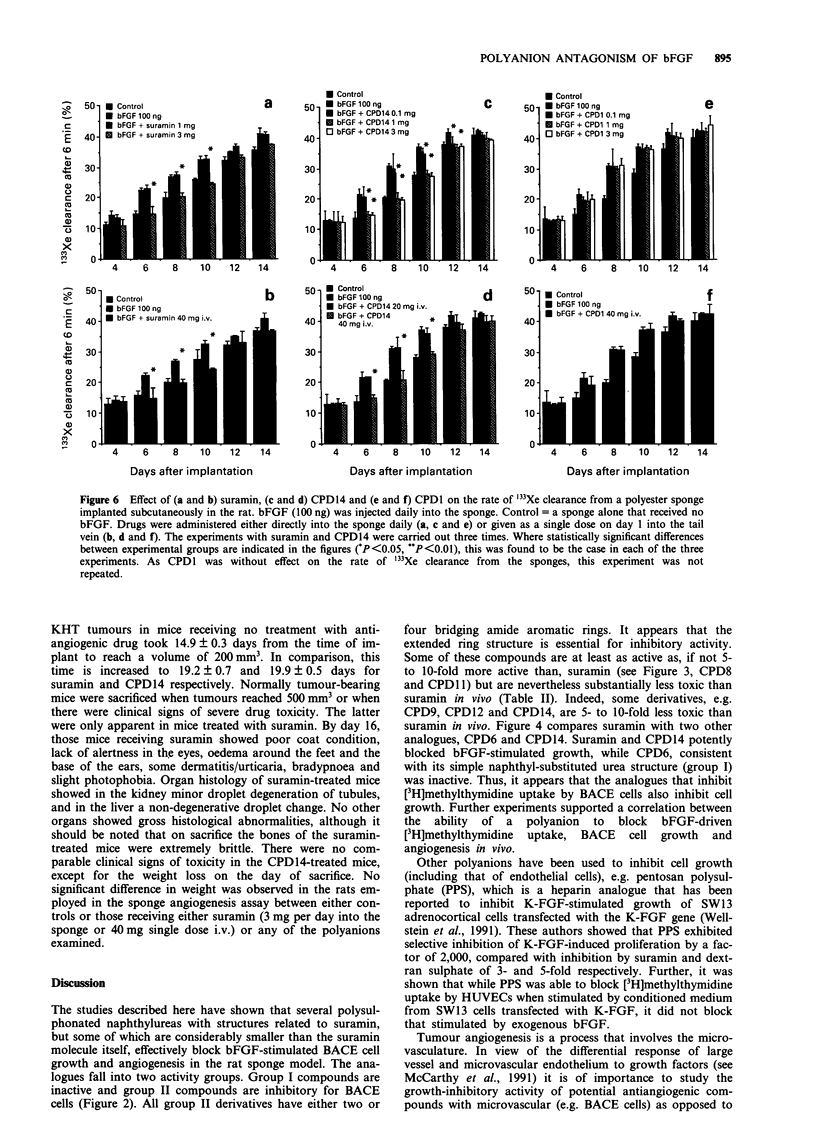
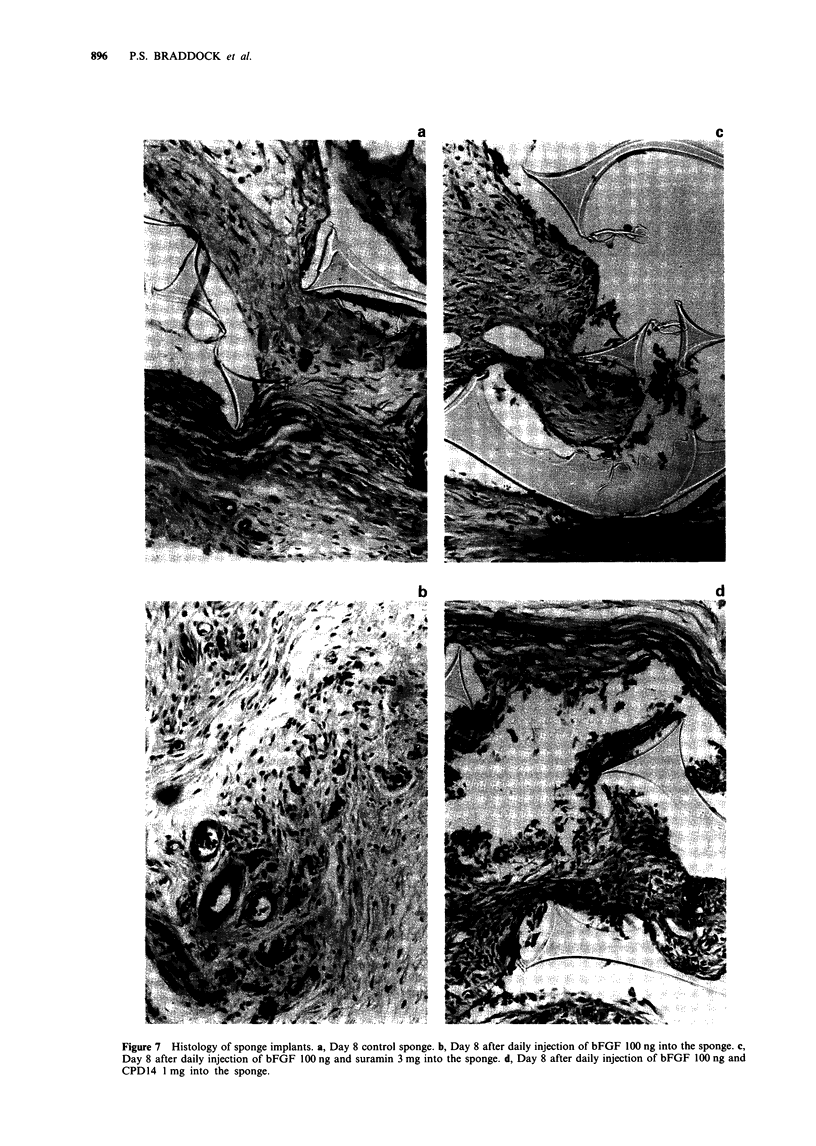
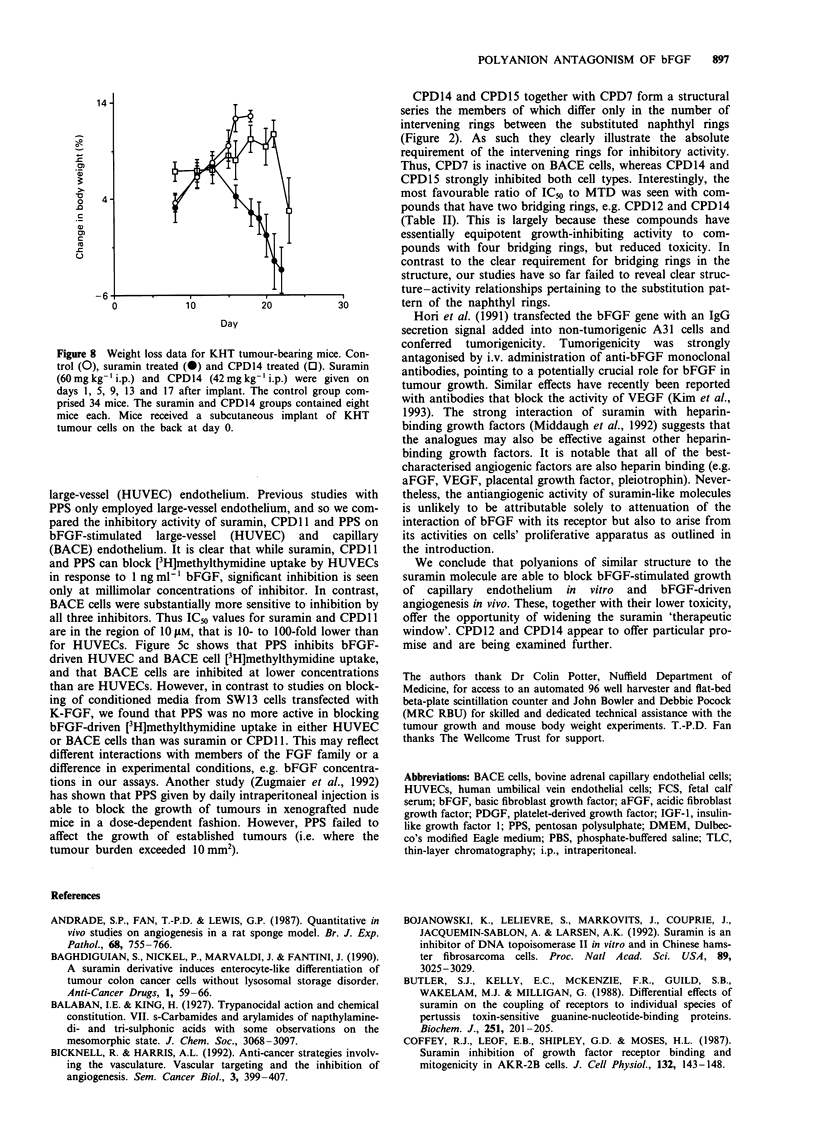
Abstract
The ability of a series of polysulphonated naphthylureas structurally related to suramin to inhibit basic fibroblast growth factor (bFGF) or serum-stimulated growth of endothelial cells [either large vessel, human umbilical vein endothelial cells (HUVEC) or microvascular, bovine adrenal capillary endothelial (BACE) cells] and angiogenesis in vivo has been examined. The polyanions encompassed two main structural variations, namely the number of aromatic amide groups intervening between two terminal naphthyl rings and/or variation in the substitution pattern of the naphthyl rings. The polyanions were either inactive (group I) or inhibited (group II) bFGF-stimulated uptake of [3H]methylthymidine by BACE cells. Group I compounds shared a common structural feature in that they were simple binaphthyl-substituted ureas. In contrast, group II compounds all had an extended multiple ring structure with at least two aromatic groups intervening between the two terminal naphthyl rings. Compounds with either two or four intervening groups were equipotent in blocking bFGF in vitro. However, compounds with two bridging aromatic groups were 5- to 10-fold less toxic than suramin in mice, suggesting a potential for an improved therapeutic ratio. The ability of the polyanions to block bFGF-driven endothelial cell proliferation in vitro correlated with antiangiogenic activity in vivo as shown by use of the rat sponge angiogenesis model. These observations could substantially widen the anti-tumour therapeutic opportunities for this class of compound.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade S. P., Fan T. P., Lewis G. P. Quantitative in-vivo studies on angiogenesis in a rat sponge model. Br J Exp Pathol. 1987 Dec;68(6):755–766. [PMC free article] [PubMed] [Google Scholar]
- Baghdiguian S., Nickel P., Marvaldi J., Fantini J. A suramin derivative induces enterocyte-like differentiation of human colon cancer cells without lysosomal storage disorder. Anticancer Drugs. 1990 Oct;1(1):59–66. doi: 10.1097/00001813-199010000-00011. [DOI] [PubMed] [Google Scholar]
- Bicknell R., Harris A. L. Anticancer strategies involving the vasculature: vascular targeting and the inhibition of angiogenesis. Semin Cancer Biol. 1992 Dec;3(6):399–407. [PubMed] [Google Scholar]
- Bojanowski K., Lelievre S., Markovits J., Couprie J., Jacquemin-Sablon A., Larsen A. K. Suramin is an inhibitor of DNA topoisomerase II in vitro and in Chinese hamster fibrosarcoma cells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3025–3029. doi: 10.1073/pnas.89.7.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler S. J., Kelly E. C., McKenzie F. R., Guild S. B., Wakelam M. J., Milligan G. Differential effects of suramin on the coupling of receptors to individual species of pertussis-toxin-sensitive guanine-nucleotide-binding proteins. Biochem J. 1988 Apr 1;251(1):201–205. doi: 10.1042/bj2510201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Leof E. B., Shipley G. D., Moses H. L. Suramin inhibition of growth factor receptor binding and mitogenicity in AKR-2B cells. J Cell Physiol. 1987 Jul;132(1):143–148. doi: 10.1002/jcp.1041320120. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A., Thompson R. W. Mechanisms of angiogenesis. Annu Rev Physiol. 1987;49:453–464. doi: 10.1146/annurev.ph.49.030187.002321. [DOI] [PubMed] [Google Scholar]
- Denekamp J., Hobson B. Endothelial-cell proliferation in experimental tumours. Br J Cancer. 1982 Nov;46(5):711–720. doi: 10.1038/bjc.1982.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J., Harris A. L., Bicknell R. Isolation and properties in culture of human adrenal capillary endothelial cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):903–908. doi: 10.1016/0006-291x(91)91503-5. [DOI] [PubMed] [Google Scholar]
- Hensey C. E., Boscoboinik D., Azzi A. Suramin, an anti-cancer drug, inhibits protein kinase C and induces differentiation in neuroblastoma cell clone NB2A. FEBS Lett. 1989 Nov 20;258(1):156–158. doi: 10.1016/0014-5793(89)81639-4. [DOI] [PubMed] [Google Scholar]
- Hori A., Sasada R., Matsutani E., Naito K., Sakura Y., Fujita T., Kozai Y. Suppression of solid tumor growth by immunoneutralizing monoclonal antibody against human basic fibroblast growth factor. Cancer Res. 1991 Nov 15;51(22):6180–6184. [PubMed] [Google Scholar]
- Hosang M. Suramin binds to platelet-derived growth factor and inhibits its biological activity. J Cell Biochem. 1985;29(3):265–273. doi: 10.1002/jcb.240290310. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch K. D., Hunsmann G., Hartmann H., Nickel P. Inhibition of human immunodeficiency virus type I reverse transcriptase by suramin-related compounds. J Gen Virol. 1987 Aug;68(Pt 8):2183–2192. doi: 10.1099/0022-1317-68-8-2183. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993 Apr 29;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Kopp R., Pfeiffer A. Suramin alters phosphoinositide synthesis and inhibits growth factor receptor binding in HT-29 cells. Cancer Res. 1990 Oct 15;50(20):6490–6496. [PubMed] [Google Scholar]
- La Rocca R. V., Stein C. A., Danesi R., Jamis-Dow C. A., Weiss G. H., Myers C. E. Suramin in adrenal cancer: modulation of steroid hormone production, cytotoxicity in vitro, and clinical antitumor effect. J Clin Endocrinol Metab. 1990 Aug;71(2):497–504. doi: 10.1210/jcem-71-2-497. [DOI] [PubMed] [Google Scholar]
- La Rocca R. V., Stein C. A., Myers C. E. Suramin: prototype of a new generation of antitumor compounds. Cancer Cells. 1990 Apr;2(4):106–115. [PubMed] [Google Scholar]
- Laitinen L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem J. 1987 Apr;19(4):225–234. doi: 10.1007/BF01680633. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Bicknell R. Responses of pertussis toxin-treated microvascular endothelial cells to transforming growth factor beta 1. No evidence for pertussis-sensitive G-protein involvement in TGF-beta signal transduction. J Biol Chem. 1992 Oct 25;267(30):21617–21622. [PubMed] [Google Scholar]
- McCarthy S. A., Kuzu I., Gatter K. C., Bicknell R. Heterogeneity of the endothelial cell and its role in organ preference of tumour metastasis. Trends Pharmacol Sci. 1991 Dec;12(12):462–467. doi: 10.1016/0165-6147(91)90637-8. [DOI] [PubMed] [Google Scholar]
- Middaugh C. R., Mach H., Burke C. J., Volkin D. B., Dabora J. M., Tsai P. K., Bruner M. W., Ryan J. A., Marfia K. E. Nature of the interaction of growth factors with suramin. Biochemistry. 1992 Sep 22;31(37):9016–9024. doi: 10.1021/bi00152a044. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Quarto N. Transformation of NIH 3T3 cells with basic fibroblast growth factor or the hst/K-fgf oncogene causes downregulation of the fibroblast growth factor receptor: reversal of morphological transformation and restoration of receptor number by suramin. J Cell Biol. 1989 Nov;109(5):2519–2527. doi: 10.1083/jcb.109.5.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., DeChavigny A., Johnson C. E., Hamada J., Stein C. A., Nicolson G. L. Suramin. A potent inhibitor of melanoma heparanase and invasion. J Biol Chem. 1991 May 25;266(15):9661–9666. [PubMed] [Google Scholar]
- Olander J. V., Connolly D. T., DeLarco J. E. Specific binding of vascular permeability factor to endothelial cells. Biochem Biophys Res Commun. 1991 Feb 28;175(1):68–76. doi: 10.1016/s0006-291x(05)81201-x. [DOI] [PubMed] [Google Scholar]
- Pesenti E., Sola F., Mongelli N., Grandi M., Spreafico F. Suramin prevents neovascularisation and tumour growth through blocking of basic fibroblast growth factor activity. Br J Cancer. 1992 Aug;66(2):367–372. doi: 10.1038/bjc.1992.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M., Richard M. Suramin blockade of insulinlike growth factor I-stimulated proliferation of human osteosarcoma cells. J Natl Cancer Inst. 1990 Aug 15;82(16):1349–1352. doi: 10.1093/jnci/82.16.1349. [DOI] [PubMed] [Google Scholar]
- Spigelman Z., Dowers A., Kennedy S., DiSorbo D., O'Brien M., Barr R., McCaffrey R. Antiproliferative effects of suramin on lymphoid cells. Cancer Res. 1987 Sep 1;47(17):4694–4698. [PubMed] [Google Scholar]
- Walz T. M., Abdiu A., Wingren S., Smeds S., Larsson S. E., Wasteson A. Suramin inhibits growth of human osteosarcoma xenografts in nude mice. Cancer Res. 1991 Jul 1;51(13):3585–3589. [PubMed] [Google Scholar]
- Wellstein A., Zugmaier G., Califano J. A., 3rd, Kern F., Paik S., Lippman M. E. Tumor growth dependent on Kaposi's sarcoma-derived fibroblast growth factor inhibited by pentosan polysulfate. J Natl Cancer Inst. 1991 May 15;83(10):716–720. doi: 10.1093/jnci/83.10.716. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Tremble P. M., Lavin M. F., Sunday M. E. Platelet-derived growth factor receptors form a high affinity state in membrane preparations. Kinetics and affinity cross-linking studies. J Biol Chem. 1984 Apr 25;259(8):5287–5294. [PubMed] [Google Scholar]
- Zugmaier G., Lippman M. E., Wellstein A. Inhibition by pentosan polysulfate (PPS) of heparin-binding growth factors released from tumor cells and blockage by PPS of tumor growth in animals. J Natl Cancer Inst. 1992 Nov 18;84(22):1716–1724. doi: 10.1093/jnci/84.22.1716. [DOI] [PubMed] [Google Scholar]