Abstract
Pancreatic lymph node-derived CD4+CD25+ T regulatory (Treg) cells inhibit in situ differentiation of islet-reactive CD8+ T cells into cytotoxic T lymphocytes, thereby preventing diabetes progression. The mechanism by which these Treg cells suppress anti-islet CD8+ T cells is unknown. Here, we show by using a CD8+ T cell-mediated model of type 1 diabetes that transforming growth factor (TGF)-β–TGF-β receptor signals are critical for CD4+CD25+ Treg cell regulation of autoreactive islet-specific cytotoxic T lymphocytes. Transgenic expression of tumor necrosis factor α from birth to 25 days of age in the islets of B6 mice that constitutively express CD80 on their β cells results in accumulation of CD4+CD25+TGF-β+ cells exclusively in the islets and pancreatic lymph nodes, which delays diabetes progression. In contrast, expression of tumor necrosis factor α until 28 days of age prevents islet accumulation of CD4+CD25+TGF-β+ Treg cells, resulting in acceleration to diabetes. Furthermore, adoptive transfer experiments demonstrated that CD4+CD25+ Treg cells could not control naïve or activated isletreactive CD8+ T cells bearing a dominant negative TGF-β receptor type II. Our data demonstrate that, in vivo, TGF-β signaling in CD8+ T cells is critical for CD4+CD25+ Treg cell suppression of isletreactive CD8+ T cells in type 1 diabetes.
In recent years, a unique population of T cells that egress from the thymus 3 days after birth have been shown to have multiple immunoregulatory properties: they suppress activation of autoaggressive T and B cells (1–3), promote tolerance of allogenic grafts (4, 5), suppress antitumor immune mechanisms (6, 7), and prevent clearance of certain persistent bacterial and viral infections. These T regulatory (Treg) cells are characterized by the expression of CD4, the IL-2 receptor α-chain (CD25), cytotoxic T lymphocyte antigen (CTLA-4) (8, 9), and glutocorticoidinduced tumor necrosis factor (TNF) receptor (GITR) (10, 11). Because of the immunoregulatory role of CD4+CD25+ Treg cells, investigations have centered on identifying the molecules and signal pathways that are critical for their recruitment, activation, and function at the sight of inflammation. This research is particularly relevant for autoimmune diseases where decreased numbers, or function, of CD4+CD25+ Treg cells have been linked to the development of type 1 diabetes (T1D) (12, 13), experimental autoimmune encephalitis (14), and autoimmune gastritis (8, 15). To date, IL-2 (16), CTLA-4 (8, 9), CD28 (13), CD154 (17), TNF-related cytokine induced molecule (TRANCE) (12), GITR (10, 11), inducible costimulator (ICOS) (18), and CCL4 (3) have all been associated with CD4+CD25+ Treg cell activation and/or recruitment to the site of inflammation.
The molecules and signal pathways that contribute to the suppressor function of CD4+CD25+ Treg cells in vivo are less well characterized. Although the suppressive cytokines IL-10 and transforming growth factor β (TGF-β) have been shown to be important for the Treg cell control of autoaggressive CD4+ T cells in inflammatory bowel disease (19, 20), it is unclear whether these cytokines participate in Treg cell control of autoaggressive CD8+ T cell-mediated autoimmune diseases, like T1D. Recently, Nakamura et al. (21) have shown that in vitro-stimulated CD4+CD25+ Treg cells both express on their membrane and secrete TGF-β. It is not known whether in vivo CD4+CD25+ Treg cells similarly express TGF-β. Indeed, a recent study (22) has argued against a role for TGF-β in Treg cell function.
Previously, we developed a murine model for T1D in which the insulin-secreting β cells of the islets of Langerhans are destroyed by CD8+ T cell-mediated mechanisms (12, 23). In these mice, termed tetracycline (Tet-TNF-α/CD80) mice, islet-specific expression of TNF-α is controlled by a doxycycline-responsive transcriptional on/off switch, and the costimulatory molecule CD80 is constitutively controlled by the rat insulin promoter (RIP) type II. Selective expression of TNF-α from birth to 28 days of age results in rapid progression to diabetes, whereas expression of TNF-α from birth to 25 days of age delays disease development (23). Recently, we showed that delay in diabetes progression is related to the accumulation of CD4+CD25+ T cells specifically in the pancreatic lymph node (PLN) and islets (12). Adoptive transfer studies showed these PLN-accumulating CD4+CD25+ T cells to be extremely potent regulatory cells, with only 2,000 cells required to prevent diabetes progression.
Here we use Tet-TNF-α/CD80 mice to further characterize the molecules and signal pathways required for CD4+CD25+ Treg function in vivo. We show that delay in diabetes progression correlates with the accumulation of TGF-β1-expressing CD4+CD25+ T cells in the PLN and islets, but not in lymphoid organs. Furthermore, by using a series of adoptive transfers, we show that anti-islet CD8+ T cells bearing a dominant negative TGF-β receptor type II transgene (dnTGFβRII tg) cannot be controlled by CD4+CD25+ Treg cells in vivo.
Our data provide insights into the mechanism by which CD4+CD25+ Treg cells regulate autoaggressive CD8+ T cells in vivo, and suggest that manipulation of the TGF-β–TGF-βRII pathway may offer therapeutic alternatives for T1D and, hopefully, other autoimmune diseases.
Materials and Methods
Mice. Tet-TNF-α C57BL/6 (B6) (23), RIP-CD80 B6 (24), and dnTGFβRII B6 (25) mice have been described elsewhere. For TNF-α repression, mice were fed doxycycline-supplemented food (2.3 g/kg; Bioserv, Frenchtown, NJ). All mice were maintained under specific pathogen-free conditions.
Cells and Tissue Preparation. For fluorescence-activated cell sorter (FACS) analysis, lymphocyte populations were prepared from the spleen, peripheral lymph nodes (periLNs: inguinal, axillary, and lateral axillary), and PLNs by standard procedures. Lymphocytes were prepared from the islets as described (23). Tissues were prepared for histology by fixing in 1% paraformaldehyde before embedding in paraffin.
FACS and Histology. All Abs used in FACS analysis were supplied by Becton Dickinson (Cowley, Oxford, U.K.). Prepared cells were washed two times in PBS containing 5% BSA, then were incubated with the appropriate fluorochrome-linked Ab for 25 min at 4°C. After three washes in PBS/BSA, the cells were acquired by using a FACSCalibur flow cytometer (Becton Dickinson), and were analyzed with cellquest.
For detection of insulin, 5- to 7-μm paraffin-embedded sections were incubated with guinea pig anti-insulin Abs (DAKO), followed by goat anti-guinea pig biotinylated Abs (Vector Laboratories, Peterborough, U.K.). Bound Ab was detected with FITC-conjugated streptavidin (Jackson ImmunoResearch). Nuclei were visualized after incubation with 4′,6-diamidino-2-phenylindole (DAPI).
Alternatively, tissue sections were stained with hematoxylin/eosin.
For detection of collagen fibers, processed tissue sections were stained with Masson's trichrome.
Adoptive Transfers. Lymphocytes were isolated as before and washed in PBS/BSA. After incubation with the appropriate labeled Ab, the desired cell populations were collected by using either a FACStarplus or MoFlo instrument (both from Becton Dickinson). In all cases, the cells were washed twice in sterile PBS (Invitrogen) before injection into the lateral tail vein of recipient mice.
Diabetes Detection. Diabetes was monitored by daily testing of urine for glucose by using Diastyx (Bayer, Newbury, U.K.) and was confirmed by measurement of glucose levels in the serum by using One-Touch strips (LifeScan, Mountain View, CA). Mice that had values >250 mg/dl on two consecutive occasions were deemed diabetic.
Results
Islet-Derived CD4+CD25+ Treg Cells Express Membrane TGF-β1. A recent study has suggested that expression of TGF-β1 on CD4+CD25+ Treg cells may contribute to their suppressive function in vitro (21), although another study contradicted these findings (22). It is unknown whether CD4+CD25+ Treg cells express TGF-β in vivo.
We tested whether levels of TGF-β1 on the surface of CD4+CD25+ Treg cells correlated with protection against diabetes in vivo. Tet-TNF-α/CD80 mice were induced to express TNF-α from birth to either 25 or 28 days of age, which normally results in delayed or accelerated progression to diabetes, respectively (12). Single-cell suspensions were prepared from the spleen, periLNs (inguinal, axillary, and lateral axillary), PLNs, and islets, and the cells were incubated with anti-CD4, CD25, and TGF-β1 Abs or rat IgG2aκ isotype control Abs. We determined the proportion of CD4+CD25+ versus CD4+CD25– cells from various tissues expressing TGF-β1 (Fig. 1). In all tissues examined (spleen, periLNs, PLNs, and islets), <2% of CD4+CD25– T cells expressed membrane TGF-β1, irrespective of the diabetes phenotype of the mouse (accelerated or delayed) (Fig. 1B). Similarly, <4% of CD4+CD25+ T cells isolated from the spleen or periLNs of either group of mice expressed TGF-β1. In contrast, of CD4+CD25+ T cells extracted from PLNs and the islets of mice that rapidly progressed to diabetes, 8% and 11% of the cells expressed TGF-β1, respectively, (Fig. 1B). However, the most dramatic increase in TGF-β1 expression was restricted to the CD4+CD25+ T cells present in the PLNs and islets of mice that showed delayed progression to diabetes, where 30% of PLN-derived CD4+CD25+ T cells and 57% of islet-derived CD4+CD25+ T cells were TGF-β1-positive (Fig. 1B).
Fig. 1.
CD4+CD25+TGF-β+ Treg cells congregate only in the islets of mice that showed delayed kinetics to diabetes. (A) Tet-TNF-α/CD80 mice were induced to express TNF-α from 0 to 28 or from 0 to 25 days of age. On day 32, cell suspensions were prepared from the tissues shown and incubated with anti-CD4 (GK1.5), anti-CD25 (PC61), anti-hTGF-β1 (A75–3.1), or rat IgG2aκ isotype control [for anti-(human)hTGF-β] Abs. The proportion of CD4+CD25+ cells or CD4+CD25– cells expressing TGF-β (black) versus isotype control (gray) Ab is shown. The data represent three mice examined in three independent experiments. (B) Tet-TNF-α/CD80 mice were induced to express TNF-α from 0 to 28 (filled bars) or from 0 to 25 (hatched bars) days of age. The cells were isolated on day 32 from the tissues indicated and incubated with anti-CD4, anti-CD25, and anti-hTGF-β1 Abs, and the percentage of CD4+CD25+ versus CD4+CD25– cells expressing TGF-β1 was determined by FACS. The data are presented as mean ± SD of three individual mice examined for each group after three independent experiments. (C) Cells were prepared as before, and the total percentage of CD4+ T cells that expressed both CD25 and TGF-β was calculated. The data are presented as mean ± SD of three individual mice examined for each group after three independent experiments.
Further analysis of the total percent of CD4+ T cells that expressed both CD25 and TGF-β1 demonstrated that <0.5% of the cells in the spleen or periLNs expressed both molecules for both groups of mice (Fig. 1C). In mice that rapidly became diabetic, 1.1% of PLN-derived and 1.3% of the islet-derived CD4+ T cells expressed CD25 and TGF-β1, whereas 9% of PLN-derived and 16% of islet-derived CD4+ T cells were CD25+TGF-β1+ in mice that developed disease with delayed kinetics.
These findings show that increased levels of TGF-β on CD4+ T cells are restricted to the CD4+CD25+ population of Treg cells that accumulate in the PLNs and islets, and the level of TGF-β1 expression correlates with delay in diabetes progression.
DnTGFβRII B6 Mice Have Perivascular Infiltrates in Pancreas but Do Not Develop Diabetes. To determine the importance of TGF-β–TGF-βR signals in the CD4+CD25+ Treg cell suppression of autoaggressive CD8+ T cells, we used B6 mice that bear a T cell promoter-driven dnTGFβRII (25). In dnTGFβRII tg mice, both CD4+ and CD8+ T cells spontaneously become activated and differentiate into cytokine secreting effector cells by 8–10 weeks of age. Histological examination of dnTGFβRII tg mice revealed perivascular infiltrates (data not shown), yet dnTGFβRII transgenic (tg) mice do not develop diabetes (data not shown). Thus, the inability to signal through TGF-βR on T cells in itself does not lead to breakdown in peripheral tolerance to host islet cells.
Both Naïve and Activated Autoaggressive CD8+ T Cells Require TGF-βR Signals for Regulation. Previously, we have shown that PLN-derived CD4+CD25+ Treg cells delay diabetes progression in our model (12). We tested whether these endogenous CD4+CD25+ Treg cells were capable of controlling CD8+ T cells that were deficient in TGF-βRII signaling.
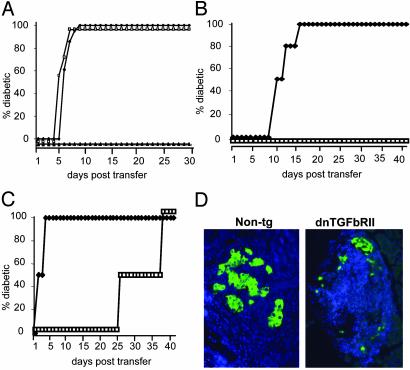
Unfractionated donor CD8+ T cells were isolated from either the PLNs or periLNs of dnTGFβRII mice or the PLNs of non-tg mice, and 3 × 104 cells were transferred into recipient Tet-TNF-α/CD80 mice that had been induced to express TNF-α from birth to 25 days of age. As shown in Fig. 2A, neither PLN-derived nor periLN-derived CD8+ T cells from dnTGFβRII tg mice were efficiently controlled by endogenous CD4+CD25+ Treg cells, with recipients developing diabetes between 5 and 9 days posttransfer. In contrast, unfractionated CD8+ T cells from non-tg mice were efficiently controlled by endogenous CD4+CD25+ Treg cells for the duration of the observation period.
Fig. 2.
TGF-βRII-deficient CD8+ T cells cannot be controlled by islet-specific regulatory mechanisms. (A) Donor CD8+ T cells were isolated from the periLNs or the PLNs of 6-week-old dnTGFβRII tg mice. As controls, CD8+ T cells were purified from the PLNs of non-tg littermates. Recipient Tet-TNF-α/CD80 mice were induced to express TNF-α from birth to 25 days of age to induce regulation. On day 30, groups of recipients were injected with 3 × 104 dnTGFβRII PLN-derived (□, n = 3), periLN-derived (♦, n = 3), or non-tg PLN-derived (▴, n = 3) CD8+ T cells. The progression to diabetes in recipient mice was monitored over a 30-day observation period by measuring blood glucose levels. Mice with readings >250 mg/dl on two consecutive occasions were deemed diabetic. (B and C) Donor CD8+ T cells were isolated from the PLN of 6-week-old dnTGFβRII or non-tg mice and incubated with anti-CD8 and anti-CD44 Abs. The CD8+CD44+ and CD8+CD44– T cell populations isolated by using FACStarplus. Recipient Tet-TNF-α/CD80 mice were induced to express TNF-α from birth to 25 days of age. On day 30 the mice were injected with either naïve (B) or activated (C) 3 × 104 dnTGFβRII (♦, n = 4) or non-tg (□, n = 4) donor cells. Diabetes progression was monitored as before. The data presented are the accumulated results from the pool of two independent experiments. (D) dnTGFβRII tg CD8+ T cells rapidly destroy islet β cells. The pancreata were removed from recipient mice of non-tg CD8+CD44+ T cells or dnTGFβRII CD8+CD44+ T cells and fixed in 1% paraformaldehyde. Five- to 7-μm paraffin-embedded sections were incubated with anti-insulin Abs (green), and nuclei were stained with DAPI (blue). The data are representative of two individual mice examined from each group.
Next, we tested whether naïve or activated CD8+ T cells from dnTGFβRII tg mice differed in their sensitivity to suppression by CD4+CD25+ Treg cells in vivo. CD8+CD44– (naïve) versus CD8+CD44+ (activated) CD8+ T cells were sorted from the PLNs of dnTGFβRII tg or non-tg littermates. Recipient Tet-TNF-α/CD80 mice were induced to express TNF-α from birth to 25 days of age to induce regulation, and on day 30, the mice were injected with 3 × 104 donor cells and monitored for diabetes progression.
Endogenous CD4+CD25+ Treg cells were poor at suppressing naïve dnTGFβRII tg CD8+CD44– T cells (Fig. 2B), because recipient mice developed diabetes within 12–16 days posttransfer. In contrast, CD4+CD25+ Treg cells prevented disease development after transfer of non-tg CD8+CD44– T cells throughout the 40-day posttransfer observation period. Transferred activated or memory non-tg CD8+CD44+ T cells were efficiently suppressed by endogenous CD4+CD25+ Treg cells, with diabetes developing between 28 and 38 days posttransfer (Fig. 2C). In contrast, transferred dnTGFβRII tg CD8+CD44+ cells resulted in diabetes development between 2 and 4 days posttransfer. Histological examination of pancreata from newly diabetic recipients that received dnTGFβRII CD8+CD44+ T cells confirmed diabetes was due to destruction of the insulin-producing β cells (Fig. 2D).
These data provide two important results: (i) in the absence of TGF-βRs on CD8+ T cells, both naïve and activated cells are refractive to regulation by CD4+CD25+ Treg cells; and (ii) in the presence of TGF-βRs, naïve T cells are more efficiently suppressed than are activated T cells by CD4+CD25+ regulatory mechanisms.
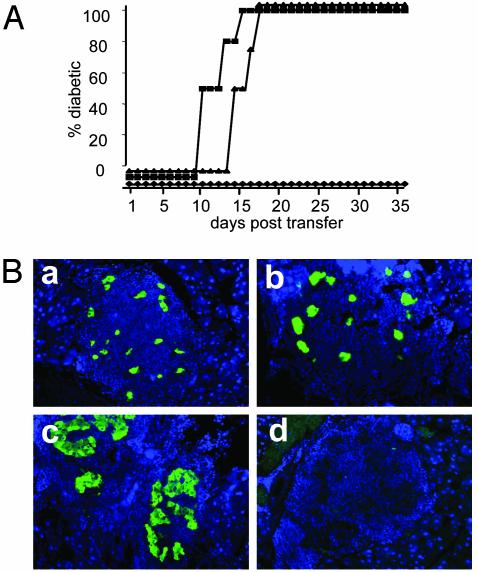
PLN-Derived CD4+CD25+ Treg Cells Cannot Control CD8+ T Cells from dnTGFβRII B6 Mice. To confirm that PLN-derived CD4+CD25+ Treg cells suppress autoreactive islet-specific CD8+ T cells by means of signaling through TGF-βRs, we performed a series of transfers. Donor CD4+CD25+ Treg cells were isolated from the PLN of regulated Tet-TNF-α/CD80 mice and were cotransferred with dnTGFβRII CD8+CD44+ tg T cells into recipient unregulated mice. As controls, groups of mice were injected with PBS or CD4+CD25+ Treg cells alone.
Mice receiving PBS rapidly developed diabetes due to endogenous islet-specific CD8+ T cell destruction of β cells as expected (Fig. 3A). Mice that received CD4+CD25+ Treg cells alone efficiently suppressed endogenous anti-islet CD8+ T cells. However, recipients of dual transfer of CD4+CD25+ Treg cells and dnTGFβRIItgCD8+ T cells developed diabetes between 10 and 15 days posttransfer. This development was slightly more rapid than that seen for mice receiving PBS, where diabetes occurred between 13 and 17 days posttransfer. Histological examination of mice receiving dual transfer of CD4+CD25+ Treg cells and dnTGFβRII tg CD8+ T cells confirmed that diabetes was due to β cell destruction (Fig. 3B). These findings confirm that CD4+CD25+ Treg cells control autoaggressive CD8+ T cells by means of TGF-βRs.
Fig. 3.
PLN-derived CD4+CD25+Treg cells fail to control islet-specific dnTGFβRII CD8+ T cells. (A) Tet-TNF-α/CD80 mice were induced to express TNF-α from birth to 25 days of age. On day 32, the PLN-derived CD4+CD25+ T cells were isolated by using MoFlo. PLN-derived cells from 6-week-old dnTGFβRII mice were incubated with rat anti-B200 and anti-CD4 Abs, and the CD8+ T cells were negatively selected by using anti-rat-coated beads. Recipient Tet-TNF-α/CD80 mice were induced to express TNF-α from birth to 28 days of age, and on day 30, groups of mice were injected with 1 × 104 CD4+CD25+ (♦, n = 3), 1 × 104 CD4+CD25+, and 3 × 104 CD8+ (▪, n = 4), or PBS (▴, n = 4). Diabetes progression was monitored as before. (B) The pancreata were removed from new diabetic recipients of PBS (a), CD4+CD25+ Treg cell and dnTGFβRII tg CD8+ T cell dual transfers (b), or CD4+CD25+ Treg cells (c), and were fixed in 1% paraformaldehyde. Five- to 7-μm paraffin-embedded sections were incubated with anti-insulin Abs (green) or isotype control Abs (d), and nuclei were stained with DAPI (blue). The data are representative of two individual mice examined from each group.
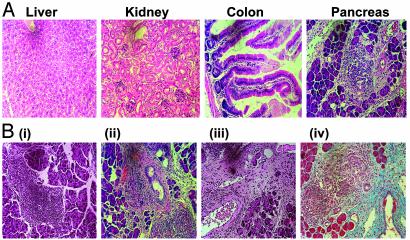
CD8+ T Cells from dnTGFβRII B6 Mice Induce Diabetes and Pancreatic Fibrosis. Over time, dnTGFβR tg mice spontaneously develop infiltrates in multiple organs including the gut, mucosa, lungs, liver, stomach, and kidney (25). We determined whether dnTGFβRII tg CD8+CD44+ T cells also infiltrated nonpancreatic tissue in Tet-TNF-α/CD80-regulated mice in addition to causing islet β cell destruction. We isolated the livers, kidneys, colon, and pancreata from new diabetic recipients that had received CD8+CD44+ T cells from the dnTGFβRII tg or non-tg mice and histologically examined the tissues.
Nonpancreatic tissue in recipients of dnTGFβRII CD8+CD44+ tg T cells (Fig. 4A) or non-tg cells (data not shown) did not have immune infiltrates. Interestingly, mice that received dnTGFβRII tg CD8+CD44+ T cells exhibited both β cell destruction and pancreatic disorganization. In contrast, although recently diabetic recipients of non-tg CD8+CD44+ T cells showed evidence of islet infiltration and β cell destruction (Fig. 4B), the exocrine pancreas was largely intact. In some cases, mice that were diagnosed as developing diabetes 2 days posttransfer were maintained on insulin for 2 more days, after which the pancreas was removed for examination. As shown in Fig. 4B, the longer the mice were diabetic, the more extensive the pancreas disorganization became. Masson's trichrome staining for collagen confirmed that this disorganization was due to fibrosis of the exocrine tissue.
Fig. 4.
Activated dnTGFβRII CD8+CD44+ T cells induce diabetes and pancreatitis. (A) Recipients of dnTGFβRII CD8+CD44+ T cells that developed diabetes 2 days posttransfer of the donor cells were killed, and the liver, kidney, colon, and pancreas were removed. After fixation in 10% formalin, 5- to 7-μm sections of paraffin-embedded tissue were stained with hematoxylin (nucleusstaining purple) and eosin (cytoplasmic-staining pink). The data are representative of four individual mice examined. (B) The pancreata were removed from new diabetic recipients of non-tg (i) or dnTGFβRII (ii) CD8+CD44+ T cells, and tissue sections were stained with hematoxylin/eosin as before. The data are representative of two individual mice examined from each group. Alternatively, recipients of dnTGFβRII CD8+CD44+ T cells that became diabetic 2 days posttransfer were treated with 2 units/ml insulin s.c. for 48 h, after which the pancreas was removed. Tissue sections were stained either with hematoxylin/eosin (iii) or Masson's trichrome (iv). The data represent two individual mice examined.
Diabetic recipients of naïve dnTGFβRII tg CD8+CD44– T cells also showed some evidence of pancreatic fibrosis, but it was relatively minor in comparison to that seen in recipients of activated dnTGFβRII CD8+CD44+ T cells (data not shown).
Thus, CD8+ T cells incapable of responding to TGF-β signals rapidly target the islets, but not nonpancreatic tissue, for destruction and subsequently promote the development of pancreatic fibrosis.
Activated CD8+ T Cells Deficient in TGF-βR Signaling Are Not Inherently Autoaggressive. We next tested whether CD8+CD44+ T cells from dnTGFβRII destroyed β cells in mice that expressed either CD80 or TNF-α in their islets.
CD8+CD44+ T cells were sorted from the PLN as before, and 3 × 104 cells were injected into either RIP-CD80 (24) or Tet-TNF-α (26) mice. In the latter group, the mice were induced to express TNF-α from birth to 25 days of age similarly to that of double-tg Tet-TNF-α/CD80 mice. As a control, regulated Tet-TNF-α/CD80 recipients were included in the assay. In all cases, the recipients were monitored for diabetes progression.
As shown in Fig. 5, RIP-CD80 tg mice did not develop diabetes over a 100-day observation period. The RIP-CD80 recipients also failed to develop islet infiltrates, suggesting that the donor cells did not traffic to the islets in these mice (data not shown). Similarly, single-tg Tet-TNF-α mice did not develop diabetes throughout the observation period. This finding contrasted starkly with double-tg Tet-TNF-α/CD80 mice that developed diabetes as before.
Fig. 5.
Activated dnTGFβRII CD8+ T cells fail to induce diabetes in RIP-CD80 and Tet-TNF-α single-tg mice. CD8+CD44+ T cells were prepared for the PLN of donor dnTGFβRII mice as before. Tet-TNF-α and Tet-TNF-α/CD80 mice were induced to express TNF-α from birth to 25 days of age. In all cases, 5-week-old recipient RIP-CD80 (▴, n = 6), Tet-TNFα (□, n = 6), or Tet-TNF-α/CD80 (♦, n = 3) mice were injected with 3 × 104 donor cells. Diabetes progression was monitored as before.
Thus, the autoaggressive potential of CD8+ T cells from dnTGFβRII mice is restricted to animal models where ongoing islet-specific autoaggressive and regulatory mechanisms are operating side by side.
Discussion
The mechanisms by which CD4+CD25+ Treg cells control autoaggressive CD8+ T cells in vivo has, to date, remained elusive. Here we have shown, in a model of T1D, that these cells may suppress islet-specific CD8+ T cells either directly through TGF-β–TGF-βR interaction between the Treg cell and the autoaggressive cell or indirectly, e.g., though the antigen-presenting cell (APC).
These hypotheses were first suggested by the increase in TGF-β expression on the surface of islet-derived CD4+CD25+ Treg cells that accumulated in the islets of mice that developed diabetes with delayed kinetics. We have previously shown that B6 mice that coexpress CD80 and TNF-α in their islets develop diabetes rapidly or slowly, depending on whether TNF-α is expressed from birth to 28 days or 25 days, respectively. Delay in diabetes progression is characterized by a significant increase in CD4+CD25+ Treg cells, specifically in the PLNs and islets (≈13% of CD4+ T cells are CD25+ in mice that exhibit acceleration to disease, whereas ≈28% of the CD4+ T cells are CD25+ in mice that develop disease with delayed kinetics). Our present findings extend this hypothesis to show that CD4+CD25+ Treg cells residing only in the PLNs and islets of mice that develop disease with delayed kinetics express TGF-β1.
Interestingly, the percentage of TGF-β1-positive cells within the CD4+CD25+ T cell fraction differs with respect to the diabetic phenotype of the mice examined. Whereas only 8% of CD4+CD25+ T cells in the PLN and 11% of CD4+CD25+ T cells in the islets of Tet-TNF-α/CD80 mice that rapidly progress to diabetes expressed TGF-β1, this value increased to 30% and 57% for CD4+CD25+ T cells in the PLNs and islets, respectively, of mice that developed disease with delayed kinetics. Thus, both the number of islet-derived CD4+CD25+ Treg cells and the percentage of TGF-β expression on those Treg cells are influenced by the duration of TNF-α expression. The differences in TGF-β1 levels on the surface of CD4+CD25+ Treg cells in the PLN and islets versus the periLNs of mice that develop diabetes with delayed kinetics may reflect the activation status of the Treg cell at different anatomical sites. Thus, the APCs and the environment in the inflamed islet and the PLN may be particularly adept at providing the necessary signals for the activation of CD4+CD25+ Treg cells, and the subsequent up-regulation in TGF-β1 expression. In contrast, lymph nodes distal from the site of inflammation do not contain the necessary stimuli that promote activation of CD4+CD25+ Treg cells, and, as a consequence, TGF-β1 levels on Treg cells remain basal.
The expression of TGF-β on Treg cell has been the source of controversy. Whereas Nakamura et al. (21) found TGF-β expression on murine Treg cells [and others report TGF-β-expressing Treg cells in humans (27, 28)], Piccirillo et al. (22), found neither TGF-β expression nor a role for TGF-β in Treg cells functioning in vitro after anti-CD3 stimulation. Our results may help resolve this controversy. We find that TGF-β expression on Treg cells is specific to the islets of mice undergoing profound islet inflammation. Importantly, TGF-β expression was not observed in other lymphoid tissues, confirming the results of Piccirillo et al. (22). Thus, in vivo TGF-β levels on Treg cells may be influenced by the environment in which the cells reside. This finding in turn may be influenced by the type of autoaggressive cells present and the mechanisms required to control them. Therefore, it will be interesting to see whether other autoimmune diseases require, like T1D, TGF-β–TGF-βR signals for Treg cell control of autoaggressive cells.
We further substantiated the hypothesis that TGF-β–TGF-βR interactions are critical for controlling anti-islet CD8+ T cells by demonstrating that dnTGFβRII tg CD8+ T cells could not be suppressed by either exogenous or endogenous CD4+CD25+ Treg cells. Although both naïve and activated dnTGFβRII tg CD8+ T cells failed to be efficiently regulated, recipients of naïve dnTGFβRIItgCD8+ T cells progressed to diabetes slightly later than did recipients of activated dnTGFβRII tg CD8+ T cells. These kinetic differences may reflect the need for naïve cell migration to the PLN and activation in response to islet antigen present on PLN-residing APCs, followed by subsequent migration to the islets. In contrast, the highly activated phenotype of dnTGFβRII tg CD8+CD44+ T cells should allow them to migrate directly to the islet. Alternatively, the rapid development of diabetes after transfer of activated versus naïve dnTGFβRII tg CD8+ T cells may simply reflect a higher frequency of islet-reactive cells in the CD8+CD44+ fraction. It could be argued that CD8+ T cells from dnTGFβRII tg mice are intrinsically more prone to activation, and, as a result, Treg cells are unable to regulate these autoaggressive CD8+ T cells through any effector mechanism; in other words, the data may not indicate that the effector mechanism of Treg cell suppression of autoaggressive CD8+ T cells in vivo is TGF-β-mediated. Our in vitro data argue against this possibility. By using ovalbumin-specific OT-1 CD8+ T cell receptor (TcR) tg T cells, we established that OT-1 CD8+ TcR Tg T cells show no difference in antigenic responsiveness to a range of ovalbumin peptides, irrespective of whether or not the cells expressed the dnTGFβRII tg (data not shown). Furthermore, in vivo the activation of OT-1 CD8+ TcR tg T cells bearing the dnTGFβRII tg was shown to be antigen-driven (data not shown). Thus, the difference between wild-type CD8+ T cells and dnTGFβRII CD8+ tg T cells in vivo is de facto a consequence of the response to the TGF-β+ environment.
Interestingly, transfer experiments demonstrated that naïve non-tg CD8+ T cells were easier to regulate than were activated non-tg CD8+ T cells. The mechanism(s) by which CD4+CD25+ Treg cells fail to maintain suppression of non-tg CD8+CD44+ T cells as efficiently as non-tg CD8+CD44– T cells are unknown. Nevertheless, it is attractive to consider that, because of the chronic inflammation present in the islets, the TGF-β–TGF-βR pathway on activated anti-host CD8+ T cells may naturally become compromised.
Another striking finding was that the dnTGFβRII tg CD8+CD44+ T cells induced both diabetes and fibrosis of the exocrine pancreas. Time course immunohistochemical analysis for insulin production determined that β cell destruction preceded fibrosis. It is unknown why fibrosis occurs. One potential explanation is that the dnTGFβRII tg CD8+CD44+ T cells induce the CD4+CD25+ Treg cells to overproduce TGF-β, promoting fibrosis. Alternatively, the fibrosis may result from prolonged inflammation in the pancreas (29, 30). Unlike dnTGFβRII tg mice, recipients of CD8+CD44+ dnTGFβRII tg T cells (containing T cells of many specificities) did not have multiple organ infiltrates. This result could not be solely attributed to the rapid kinetics that the recipient mice developed disease, thereby hindering long-term analysis of nonpancreatic tissue, because we never saw any infiltrates in nonpancreatic tissue isolated from Tet-TNF-α or RIP-CD80 single-tg mice 11 weeks posttransfer of the aggressive cells. Again, it is possible that a higher frequency of islet-reactive CD8+ T cells exist in the donor fraction that contributes to the phenotype seen. An alternative possibility is that multiple organ infiltration requires defects in the TGFβRII signal pathway for both CD4+ and CD8+ T cells, as seen for dnTGFβRII tg mice.
We showed that dnTGFβRII tg T cells were aggressive only in the Tet-TNF-α/CD80-regulated model. Indeed, transfer of dnTGFβRII tg CD8+ T cells into either RIP-CD80 or Tet-TNF-α single-tg mice induced neither diabetes nor pancreatic fibrosis. Thus, neither the presence of CD80 on β cells nor the presence of inf lammation on their own can transform dnTGFβRII tg CD8+ T cells into β cell killers, whereas the combination of the two conditions is highly conductive for the potentiation of autoaggressive behavior, even in the presence of potent Treg cells.
In conclusion, we have shown that CD4+CD25+ Treg cells control autoaggressive islet-specific CD8+ T cells through TGF-β–TGF-βR signals in vivo. Determining whether TGF-β-expressing Treg cells specifically interact with TGFβRII on CD8+ T cells in situ to control them or suppress CD8+ T cell responses by means of modulation of intra-islet APCs will be clarified by the development of new tg strains where only the APCs or Treg cells are deficient in TGF-β. Nevertheless, because of our evidence that disease acceleration is linked to expression levels of TGF-β on Treg cells, and the well documented reports that suppression of effector responses requires cell:cell contact (21, 31, 32), we favor the former hypothesis. Future studies to determine the mechanisms by which TGF-β–TGF-βR signals can be manipulated may offer therapeutic targets for treatment of T1D and, hopefully, other autoimmune disease.
Acknowledgments
We thank Kerry Payne and Nicola Armitage for technical assistance, Tom Taylor and Andrew Riddle for sorting expertise, and Charles River Laboratories for rederivation of Tet-TNF-α and RIP-CD80 mice. This work was supported by the Juvenile Diabetes Research Foundation and Wellcome Trust Grant 061861 (to E.A.G.), the American Diabetes Association (R.A.F.), and National Institutes of Health Grant R01 DK51665 (to R.A.F.). R.A.F. is an investigator of the Howard Hughes Medical Institute.
Abbreviations: Treg, T regulatory; T1D, type 1 diabetes; TGF-β, transforming growth factor β; TNF, tumor necrosis factor; dnTGFβRII, dominant negative TGF-β receptor type II; tg, transgenic; Tet, tetracycline; RIP, rat insulin promoter; PLN, pancreatic lymph node; periLN, peripheral lymph node; FACS, fluorescence-activated cell sorter; APC, antigen-presenting cell.
References
- 1.Shevach, E. M. (2001) J. Exp. Med. 193, F41–F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens, L. A., Mottet, C., Mason, D. & Powrie, F. (2001) Eur. J. Immunol. 31, 1247–1254. [DOI] [PubMed] [Google Scholar]
- 3.Bystry, R. S., Aluvihare, V., Welch, K. A., Kallikourdis, M. & Betz, A. G. (2001) Nat. Immunol. 2, 1126–1132. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann, P., Ermann, J., Edinger, M., Fathman, C. G. & Strober, S. (2002) J. Exp. Med. 196, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J., Huoam, C., Plain, K., He, X. Y., Hodgkinson, S. J. & Hall, B. M. (2001) Transplant. Proc. 33, 163–164. [DOI] [PubMed] [Google Scholar]
- 6.Somasundaram, R., Jacob, L., Swoboda, R., Caputo, L., Song, H., Basak, S., Monos, D., Peritt, D., Marincola, F., Cai, D., et al. (2002) Cancer Res. 62, 5267–5272. [PubMed] [Google Scholar]
- 7.Tanaka, H., Tanaka, J., Kjaergaard, J. & Shu, S. (2002) J. Immunother. 25, 207–217. [DOI] [PubMed] [Google Scholar]
- 8.Read, S., Malmstrom, V. & Powrie, F. (2000) J. Exp. Med. 192, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi, T., Tagami, T., Yamazaki, S., Uede, T., Shimizu, J., Sakaguchi, N., Mak, T. W. & Sakaguchi, S. (2000) J. Exp. Med. 192, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHugh, R. S., Whitters, M. J., Piccirillo, C. A., Young, D. A., Shevach, E. M., Collins, M. & Byrne, M. C. (2002) Immunity 16, 311–323. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. (2002) Nat. Immunol. 3, 135–142. [DOI] [PubMed] [Google Scholar]
- 12.Green, E. A., Choi, Y. & Flavell, R. A. (2002) Immunity 16, 183–191. [DOI] [PubMed] [Google Scholar]
- 13.Salomon, B., Lenschow, D. J., Rhee, L., Ashourian, N., Singh, B., Sharpe, A. & Bluestone, J. A. (2000) Immunity 12, 431–440. [DOI] [PubMed] [Google Scholar]
- 14.Furtado, G. C., Olivares-Villagomez, D., Curotto de Lafaille, M. A., Wensky, A. K., Latkowski, J. A. & Lafaille, J. J. (2001) Immunol. Rev. 182, 122–134. [DOI] [PubMed] [Google Scholar]
- 15.Kullberg, M. C., Jankovic, D., Gorelick, P. L., Caspar, P., Letterio, J. J., Cheever, A. W. & Sher, A. (2002) J. Exp. Med. 196, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malek, T. R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. (2002) Immunity 17, 167–178. [DOI] [PubMed] [Google Scholar]
- 17.Kumanogoh, A., Wang, X., Lee, I., Watanabe, C., Kamanaka, M., Shi, W., Yoshida, K., Sato, T., Habu, S., Itoh, M., et al. (2001) J. Immunol. 166, 353–360. [DOI] [PubMed] [Google Scholar]
- 18.Akbari, O., Freeman, G. J., Meyer, E. H., Greenfield, E. A., Chang, T. T., Sharpe, A. H., Berry, G., DeKruyff, R. H. & Umetsu, D. T. (2002) Nat. Med. 8, 1024–1032. [DOI] [PubMed] [Google Scholar]
- 19.Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. (1999) J. Exp. Med. 190, 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuss, I. J., Boirivant, M., Lacy, B. & Strober, W. (2002) J. Immunol. 168, 900–908. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura, K., Kitani, A. & Strober, W. (2001) J. Exp. Med. 194, 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccirillo, C. A., Letterio, J. J., Thornton, A. M., McHugh, R. S., Mamura, M., Mizuhara, H. & Shevach, E. M. (2002) J. Exp. Med. 196, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green, E. A. & Flavell, R. A. (2000) Immunity 12, 459–469. [DOI] [PubMed] [Google Scholar]
- 24.Guerder, S., Meyerhoff, J. & Flavell, R. (1994) Immunity 1, 155–166. [DOI] [PubMed] [Google Scholar]
- 25.Gorelik, L. & Flavell, R. A. (2000) Immunity 12, 171–181. [DOI] [PubMed] [Google Scholar]
- 26.Picarella, D. E., Kratz, A., Li, C. B., Ruddle, N. H. & Flavell, R. A. (1993) J. Immunol. 150, 4136–4150. [PubMed] [Google Scholar]
- 27.Annunziato, F., Cosmi, L., Liotta, F., Lazzeri, E., Manetti, R., Vanini, V., Romagnani, P., Maggi, E. & Romagnani, S. (2002) J. Exp. Med. 196, 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonuleit, H., Schmitt, E., Kakirman, H., Stassen, M., Knop, J. & Enk, A. H. (2002) J. Exp. Med. 196, 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanvito, F., Nichols, A., Herrera, P. L., Huarte, J., Wohlwend, A., Vassalli, J. D. & Orci, L. (1995) Biochem. Biophys. Res. Commun. 217, 1279–1286. [DOI] [PubMed] [Google Scholar]
- 30.Su, S. B., Motoo, Y., Xie, M. J., Miyazono, K. & Sawabu, N. (2000) Dig. Dis. Sci. 45, 151–159. [DOI] [PubMed] [Google Scholar]
- 31.Shevach, E. M., McHugh, R. S., Piccirillo, C. A. & Thornton, A. M. (2001) Immunol. Rev. 182, 58–67. [DOI] [PubMed] [Google Scholar]
- 32.Thornton, A. M. & Shevach, E. M. (2000) J. Immunol. 164, 183–190. [DOI] [PubMed] [Google Scholar]