Abstract
NF-κB is known to exert its antiviral innate immune response via the IFN-β-induced Janus kinase/signal transducers and activators of transcription pathway. However, our current studies have demonstrated that activated NF-κB is capable of directly establishing an antiviral state independent of IFN or secreted soluble factor(s) against two highly pathogenic respiratory RNA viruses. Human parainfluenza virus type 3, a mildly cytopathic virus that induced NF-κB very early during infection was converted to a virulent virus after NF-κB inhibition. In contrast, a highly cytopathic virus, human respiratory syncytial virus that induced NF-κB late during infection, was converted to a mildly cytopathic virus after NF-κB induction before virus replication. This interconversion of cytopathic phenotypes of viruses after NF-κB modulation was further shown to be independent of IFN and soluble secreted factors(s). Moreover, tumor necrosis factor α (TNF-α) and IL-1β elicited an antiviral response, which was NF-κB-dependent. Thus, NF-κB induction directly confers an essential innate antiviral response against human parainfluenza virus type 3 and respiratory syncytial virus, which is independent of IFN-inducible factor(s).
Innate immune response initiated by the infected host cells constitutes the first line of defense against foreign pathogens including viruses, before orchestrating a well organized adaptive immune response. NF-κB, a family of evolutionarily conserved transcription factors, represents an important modulator of innate and adaptive immune function required for optimal host defense (1–4). Viruses have evolved to activate NF-κB, either by double-stranded RNA intermediate or activation of Toll-like receptors (TLRs), leading to nuclear translocation of NF-κB (5–7). In the nucleus, NF-κB binds to its cognate promoter sites to activate an array of genes, including proinflammatory cytokines, chemokines, and adhesion molecules (7). These molecules are involved in initiating adaptive immunity process by recruiting immune cells to the site of infection. Apart from the adaptive immune responders, NF-κB's innate immune function is mediated by the activation of IFN-β, an important antiviral cytokine (8, 9), through which paracrine action activates the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) antiviral pathway (8, 9).
We have used two viruses, human parainfluenza virus type 3 (HPIV-3) and human respiratory syncytial virus (RSV), to study the role of NF-κB activation in conferring essential innate antiviral response in human epithelial cells. These cells facing the luminal side (e.g., intestine, lung, and airway) have direct contact with the exterior milieu and are, therefore, the initial target for majority of pathogens, including viruses (10). Both HPIV-3 and RSV, belonging to the paramyxoviridae family, are enveloped single-stranded RNA containing viruses of negative polarity that replicates in the cytoplasm (11). These viruses are important human respiratory tract pathogens, causing high morbidity among infants, children, and immunocompromised adults manifesting disease states including, pneumonia, croup, and brochiolitis (11). To date, no effective vaccine or antiviral therapy exists for either of these viruses. Therefore, elucidation of innate immune antiviral response elicited by these viruses holds significant potential for development of effective antiviral therapies against these viruses.
In this article, we report that NF-κB is capable of signaling an innate antiviral response that is independent of IFN and the well established JAK/STAT antiviral pathway. The importance of NF-κB-mediated innate response was further borne out by our observation that the temporal nature of NF-κB induction profile exhibited by RSV and HPIV-3 had direct bearing on their respective cytopathic phenotype and replication capability. Moreover, proinflammatory cytokines like tumor necrosis factor-α (TNF-α) and IL-1β exerted a potent antiviral action, which was directly dependent on the NF-κB innate antiviral pathway. The antiviral role of NF-κB against these cytoplasmic RNA viruses is discussed.
Materials and Methods
Cells and Viruses. A549, CV-1 cells, WT and IKKγ–/– mouse embryonic fibroblasts (MEFs), and human epithelial-like fibrosarcoma cells (WT and STAT-1–/– cells) were cultured as described (10, 12–15). HPIV-3, RSV, and vesicular stomatitis virus (VSV) adenoviruses (Ads) were propagated in CV-1, HepG2, BHK, and HEK cells, respectively, as described (10, 14–16).
Plaque Assay. Plaque assay was performed as described (15). To visualize the cytopathic effect, the same dilutions of medium supernatants were similarly added to CV-1 cells, and the plaques were viewed by phase contrast microscopy (×10 objective). The plaque assay data shown in the figures represents the mean number of plaque-forming units/ml from three independent experiments with similar results.
Virus Infection. A549 cells pretreated with used pyrollidine dithiocarbamate (PDTC) (Calbiochem; 50 μM) for 4 h or infected with the Ads [200 multiplicity of infection (mois)] for 16 h were infected with HPIV-3 (0.1 moi) or RSV (0.1 moi), either in the absence or presence of PDTC. After 36 h postinfection, the medium supernatants were prepared for plaque assay (15). The MEFs and fibrosarcoma cells were similarly infected with HPIV-3 and RSV (0.1 moi).
Electrophoretic Mobility-Shift Assay (EMSA) and Luciferase Assay. Nuclear extracts were prepared from HPIV-3 (3 mois), RSV (1 moi) -infected A549 cells as described (17). The nuclear extracts (8 μg of protein) were either incubated with 32P-labeled NF-κB oligonucleotide probe in the presence of preimmune/normal rabbit serum (NRS) or p65 antibody (Santa Cruz Biotechnology), or incubated with a 50× molar excess of unlabeled specific WT or mutant NF-κB oligonucleotide probe, and analyzed as described (16, 17). For luciferase assay, A549 cells were transiently transfected with plasmids containing the 2× NF-κB promoter fused to the firefly luciferase gene (16) and Renilla luciferase (for normalization of transfection efficiencies) by using lipofectin (GIBCO/BRL) as described (16). Sixteen hours posttransfection, cells were infected with either HPIV-3 or RSV (1 moi) and 8 h (for HPIV-3) or 20 h (for RSV) postinfection, the cell lysates were prepared and assayed for firefly lucifease expression as described (16). The luciferase activity was normalized to the Renilla luciferase activity (dual luciferase assay from Promega). NF-κB-luciferase-(NF-κB-Luc) transfected A549 cells were also infected with Ads [Ad-GFP, IκB super repressor (Ad-IκB-SR), and Ad-expressing dominant negative TLR adaptor protein MyD88 (Ad-DN-MyD88)] for 12 h, followed by either mock infection or infection with RSV or HPIV-3 (1 moi) for 18 and 8 h, respectively. Similarly NF-κB-Luctransfected A549 cells were infected with either Ad-GFP or Ad-IκB-SR for 12 h, followed by TNF-α or IL-1β treatment (20 ng/ml) for 2 h. The lysates from these cells were assayed for luciferase activity as described above. The luciferase assay results shown in the figures represent the average of three independent experiments and the standard deviations are shown as error bars.
Treatment of HPIV-3-Infected A549 Cells with NG-monomethyl-l-arginine (l-NMMA), Conditioned Medium (CM), or IFN-β. A549 cells pretreated with 5 mm or 25 mM l-NMMA (Oxis International, Portland, OR) for 4 h were infected with HPIV-3 (0.1 moi) for 36 h in the absence or presence of l-NMMA. To prepare the CM, A549 cells were either mock infected or infected with HPIV-3 (0.1 or 1 moi) for 24 h. The resulting medium supernatants were added to Centricon units (Centricon Plus-20, 300,000-kDa cutoff; Millipore) and centrifuged per manufacturer's direction. After centrifugation, the membrane flow-through supernatants were checked for the absence of virus by infecting fresh A549 cells. Once the absence of virus was confirmed, the mock or the HPIV-3 CMs were added to Ad-infected cells simultaneously during adsorption (2 h at 37°C) of HPIV-3 (0.1 moi). The CM was present during the course of the infection (36 h). IFN-β (2,000 units/ml) (PBL) was similarly added to Ad-infected cells simultaneously during adsorption (2 h at 37°C) of HPIV-3 (0.1 moi), and it was present during the course of the infection (36 h). The medium supernatants from l-NMMA-, CM-, or IFN-β-treated A549 cells infected with HPIV-3 were processed for plaque assay analysis.
RSV and VSV Infection of A549 or Human Fibrosarcoma Cells Pretreated with Either TNF-α or IL-1β in the Presence of Either Ad-GFP or Ad-IκB-SR. A549 or human fibrosarcoma cells were pretreated with TNF-α or IL-1β (20 ng/ml) (R & D Systems) for either 8 or 16 h. After pretreatment, the cells were infected with RSV, HPIV-3, or VSV (0.1 moi) for 36 h in the absence of these cytokines. A549 cells were also infected with Ads (Ad-GFP or Ad-IκB-SR) for 12 h, followed by TNF-α or IL-1β pretreatment (20 ng/ml) for 16 h. These cells were then infected with either RSV or VSV (0.1 moi) for 36 h in the absence of these cytokines. The medium supernatants from these cells were then subjected to plaque assay analysis on CV-1 cells.
RSV and VSV Infection of A549 or Human Fibrosarcoma Cells Pretreated with Either TNF-α or IL-1β CM in the Presence of Anti-TNF-α or Anti-IL-1β Neutralizing Antibodies, Respectively. CM obtained from untreated and TNF-α- or IL-1β-treated (20 ng/ml) A549 (8, 16, or 24 h) or human fibrosarcoma (24 h) cells were incubated with either control or respective cytokine-neutralizing antibodies (300 ng/ml; R & D Systems) overnight at 4°C. After incubation, the medium was added to fresh A549 or human fibrosarcoma cells for 16 h pretreatment before adding RSV or VSV (0.1 moi). The medium supernatants obtained from these cells after 36 h virus infection were subjected to plaque assay analysis on CV-1 cells
Results
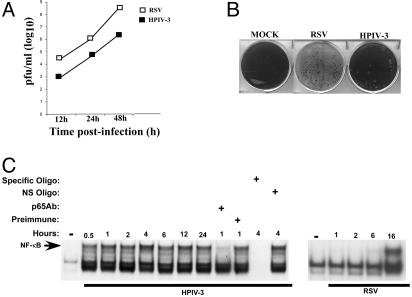
Temporal Activation of NF-κB by HPIV-3 and RSV in Human Lung Epithelial Cells. Human lung epithelial A549 cells were initially tested for susceptibility to HPIV-3 and RSV infections. We observed that HPIV-3 is significantly less cytopathic than RSV in these cells; the cells that are the primary target of these viruses during productive infection (ref. 11 and Fig. 1 A and B). A single-step growth kinetics of these viruses (0.1 moi) at 12, 24, and 48 h postinfection revealed that HPIV-3 titer was significantly lower (two logs) compared with RSV (Fig. 1 A). These differences can also be directly visualized by their ability to form syncytia; cytopathic effect analysis (Fig. 1B) of these viruses at the same dilution of medium supernatants showed few plaques for HPIV-3, whereas RSV completely disseminated the cells. These results demonstrated that HPIV-3 replicated poorly in A549 cells, whereas RSV was highly cytopathic.
Fig. 1.
Replication kinetics and NF-κB-induction profile of HPIV-3 and RSV in human lung epithelial A549 cells. (A) A single-step growth kinetics of HPIV-3 and RSV (0.1 moi) in A549 cells was determined by plaque assay analysis. (B) Cytopathic effect analysis (48 h postinfection) of HPIV-3 and RSV (0.1 moi) from infected A549 cells was determined after addition of same dilution of A549 medium supernatants to CV-1 cells for plaque assay analysis. (C) NF-κB EMSA using nuclear extracts from uninfected (–) and RSV-infected (1, 2, 6, and 16 h postinfection) A549 cells, and HPIV-3-infected (0.5–24 h postinfection) A549 cells in the absence or presence of NF-κB p65 antibody (Ab), preimmune serum, specific NF-κB unlabeled probe, or mutant NF-κB unlabeled probe (NS) as indicated.
We next investigated whether these viruses possessing widely different cytopathic phenotype also differ in their induction of NF-κB, which is an important innate immune responder (1–4). EMSA (Fig. 1C) performed with nuclear extracts obtained from RSV- or HPIV-3-infected A549 cells, revealed rapid NF-κB DNA-binding activity very early after HPIV-3 infection (30 min postinfection) before initiating its full replicative cycle (12–16 h postinfection; refs. 18 and 19). In contrast, RSV-induced NF-κB DNA-binding activity was observed considerably later (16 h) after the onset of replication (10–12 h postinfection; ref. 20), similar to that reported (21, 22). Specificity of the HPIV-3-induced NF-κB complex formation was demonstrated by super-shift analysis with p65 antibody and competition with unlabeled WT, but not mutant oligonucleotide probe (Fig. 1C).
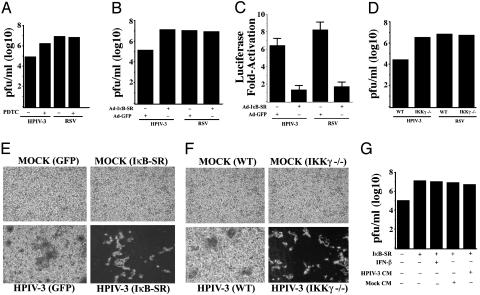
Inhibition of NF-κB Activation Results in Increased Replication and Cytopathogenicity of HPIV-3 Independent of IFN. The differential kinetics of NF-κB induction displayed by RSV and HPIV-3 raised the possibility that these viruses may have evolved to regulate the activation of NF-κB to strategically manipulate the antiviral defense mechanism exerted by the host for efficient replication, leading to higher cytopathic phenotype. To examine this possibility, we monitored the replication capability of HPIV-3 and RSV in cells where NF-κB was inactivated. By using PDTC, a general NF-κB inhibitor (ref. 23 and Fig. 2A), during infection resulted in a significant (30-fold) increase in HPIV-3 titer. In contrast, PDTC had no effect on RSV titer (Fig. 2 A). These results were further confirmed by expressing the Ad-IκB-SR (32A/36A) (16, 24) in HPIV-3-infected cells. As shown in Fig. 2B, IκB-SR expression lead to dramatic increase (100-fold) in HPIV-3 titer, whereas RSV titer remained unchanged. The Ad-IκB-SR used in these studies was functional, because Ad-IκB-SR, but not control Ad-GFP, inhibited NF-κB-Luc induction by HPIV-3 and RSV in A549 cells (Fig. 2C). Finally, virus obtained from infected WT and IKKγ–/– MEFs (12) demonstrated dramatic augmentation (100-fold) of HPIV-3, but not RSV replication in IKKγ–/– cells (Fig. 2D). The significant increase in HPIV-3 cytopathogenicity and viral titer could be clearly visualized by increased syncytia formation after NF-κB inactivation (Fig. 2 E and F). The observed conversion of mildly cytopathic HPIV-3 into a virulent form, similar to RSV, after inhibition of NF-κB, suggested an important role of NF-κB in antiviral defense. These results indicate that rapid activation of NF-κB before HPIV-3 replication constitutes an essential antiviral host defense in human lung epithelial cells, as well as in mouse fibroblasts.
Fig. 2.
Inhibition of NF-κB activation increases HPIV-3, but not RSV, replication and cytopathogenicity in an IFN- and/or soluble factor(s)-independent manner. Plaque assay analysis using medium supernatants from A549 cells mock infected or infected with either HPIV-3 or RSV in the absence or presence of PDTC (50 μM) (A), or A549 cells infected with HPIV-3 or RSV in the absence or presence of prior infection with Ads encoding the IκB-SR or GFP(B). (C) Expression of transfected NF-κB-Luc in A549 cells infected with HPIV-3 or RSV in the presence of IκB-SR or control GFP. (D) Plaque assay analysis using medium supernatants from IKKγ–/– or WT MEFs infected with either HPIV-3 or RSV. Phase contrast microscopic picture of CV-1 cells incubated with same dilutions of medium supernatants obtained from A549 cells infected with HPIV-3 and Ad-GFP or Ad-IκB-SR (E) and HPIV-3-infected WT or IKKγ–/– MEFs (F). (G) Plaque assay analysis using medium supernatants from A549 cells infected with HPIV-3 and Ad-GFP or Ad-IκB-SR and treated with IFN-β (2,000 units/ml). Similar analysis was performed with medium supernatants from A549 cells infected with HPIV-3 and Ad-GFP or Ad-IκB-SR in the presence or absence of either mock CM or HPIV-3 CM (cleared free of virus).
We next examined whether the conversion of a mildly cytopathic virus, HPIV-3, to a virulent one on NF-κB inhibition was due to the lack of IFN-β [a gene which is stimulated in HPIV-3-infected epithelial cells (25, 26)] or soluble antiviral secreted factor(s) production. As shown in Fig. 2G, addition of exogenous IFN-β (2,000 units/ml) to the NF-κB-inactivated cells at the time of HPIV-3 infection failed to inhibit the increased infectivity and cytopathogenicity of HPIV-3. Similar results were obtained after addition of IFN-α (data not shown). The potential involvement of putative secretory antiviral soluble factor(s) were also eliminated, because CM supernatant obtained from HPIV-3-infected A549 cells (cleared free of virus) or mock-infected cells added to Ad-IκB-SR- and HPIV-3-infected cells at the time of HPIV-3 infection, failed to repress the increased virus infectivity (Fig. 2G). Thus, it seems that NF-κB-mediated anti-HPIV-3 activity is conferred by establishing an intracellular antiviral state independent of IFN and/or soluble factor(s).
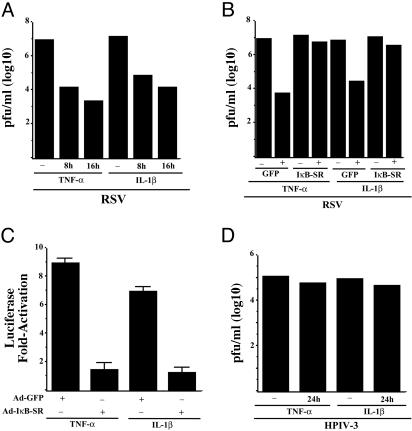
Inhibition of RSV Replication After Specific Induction of NF-κB by TNF-α or IL-1β. If indeed NF-κB is an essential innate antiviral mediator as shown above, we hypothesized that RSV may have maintained its highly cytopathic phenotype as a result of deregulating NF-κB activation by inducing it late in the replication cycle. If such induction constitutes the mechanism used by RSV to evade host's NF-κB-dependent antiviral response for preservation of its high virulence, activation of NF-κB before the replication of RSV may confer an antiviral state. To examine this possibility, we pretreated A549 cells with TNF-α (27) or IL-1β (28), which are potent inducers of NF-κB, before RSV infection. Pretreatment of cells with TNF-α or IL-1β (Fig. 3A) severely restricted RSV replication with a decrease in virus titer by 1,000-fold. However, treatment of A549 cells with TNF-α or IL-1β 10–12 h postinfection (after replication initiation) failed to restrict RSV replication (data not shown). It is important to note that in these studies, TNF-α and IL-1β were present only during the pretreatment period, but not during virus infection. These results strongly suggest that establishment of NF-κB-dependent antiviral state before infection was sufficient to restrict the replication of RSV.
Fig. 3.
Effect of TNF-α and IL-1β pretreatment on NF-κB-dependent restriction of RSV replication and cytopathogenicity. (A) Plaque assay analysis of medium supernatants from A549 cells pretreated with 20 ng/ml TNF-α or IL-1β for 8–16 h before RSV infection. (B) Plaque assay analysis of culture supernatants from IκB-SR or GFP expressing A549 cells, pretreated with either TNF-α or IL-1β for 16 h before RSV infection. (C) Expression of transfected NF-κB-Luc in A549 cells treated with either TNF-α or IL-1β (20 ng/ml for 2 h) in the presence of IκB-SR or control GFP. (D) Plaque assay analysis of medium supernatants from A549 cells pretreated with 20 ng/ml TNF-α or IL-1β for 24 h before HPIV-3 infection.
Because TNF-α and IL-1β induces additional signaling pathways (e.g., c-Jun N-terminal kinase and extracellular signal-regulated kinase) apart from NF-κB (29, 30), we investigated whether the antiviral action of TNF-α and IL-1β is specifically mediated by activated NF-κB. Inhibition of NF-κB activation after expression of IκB-SR significantly reverted the antiviral action of TNF-α and IL-1β (Fig. 3B) toward RSV. In control experiments, TNF-α or IL-1β treatment of A549 cells transfected with NF-κB-Luc led to significant induction of NF-κB activity, which was inhibited when the cells were infected with Ad-IκB-SR (Fig. 3C). These results demonstrated the potent antiviral action of TNF-α and IL-1β is indeed mediated specifically via the NF-κB signaling pathway, and the latter pathway plays an important innate antiviral role in host cells, only when it is activated before virus replication. Interestingly, TNF-α or IL-1β (Fig. 3D) pretreatment failed to exert an anti-HPIV-3 activity. Presumably, HPIV-3-mediated induction of NF-κB during the normal course of infection represents the optimal threshold value for its antiviral activity, which is not augmented further by TNF-α or IL-1β treatment.
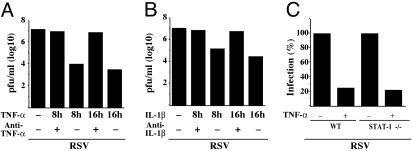
NF-κB-Dependent Antiviral Response by TNF-α and IL-1β Is Mediated Independent of IFN and Soluble Secreted Factor(s). Next, we investigated whether IFN and/or soluble factor(s) are involved in eliciting the NF-κB-dependent antiviral mechanism of TNF-α and IL-β against RSV. As shown in Fig. 4 A and B, the medium supernatant obtained from TNF-α- (Fig. 4A) or IL-1β- (Fig. 4B) treated A549 cells (cytokine CM) when treated with anti-TNF-α or anti-IL-1β neutralizing antibodies, respectively, and added to fresh A549 cells for pretreatment before virus infection, failed to inhibit RSV replication. The specificity of these cytokineneutralizing antibodies was borne out by the observation that they failed to inhibit the antiviral action of IFN and the TNF-α and IL-1β CM retained its antiviral property even in the presence of anti-IFN neutralizing antibody (data not shown).
Fig. 4.
The noninvolvement of IFN and/or soluble secretory factor(s) in mediating the NF-κB-dependent antiviral response elicited by TNF-α and IL-1β. Plaque assay analysis of medium supernatants from A549 cells infected with RSV after a 16-h pretreatment of A549 cells with medium obtained from TNF-α-(A) or IL-1β-(B) treated (8 or 16 h) A549 cells in the presence of control antibody or anti-TNF-α (A) or anti-IL-1β (B) neutralizing antibodies. (C) Plaque assay analysis using medium supernatants from WT or STAT-1–/– cells untreated or pretreated with TNF-α (20 ng/ml for 16 h) before infection with RSV. The percent infection indicated was calculated based on a ratio of number of plaques obtained in the presence of TNF-α over the number obtained from untreated cells.
The noninvolvement of soluble secreted factor(s), including IFN, in exerting the NF-κB-dependent antiviral state was further shown by using WT 2fTGH- and IFN-insensitive STAT-1 null (STAT-1–/–) U3A human epithelial-like fibrosarcoma cells (31). The lack of JAK/STAT signaling pathway was shown to have no effect on NF-κB signaling cascade induced by TNF-α treatment (32). Similar to A549 cells, TNF-α (Fig. 4C) or IL-1β (data not shown) pretreatment established an antiviral state for RSV in both WT and STAT-1–/– cells. Moreover, additional soluble secreted factor(s) were not involved during establishment of the antiviral state in WT or STAT-1–/– cells, as tested by performing similar experiments described in Fig. 4 A and B (data not shown). These results suggested that IFN and/or soluble secreted factor(s) are not involved in exerting a NF-κB-dependent antiviral state.
Use of MyD88 by HPIV-3 for NF-κB Induction. Because our results have suggested that a critical time frame of NF-κB activation in infected cells dictates the antiviral function of NF-κB, we investigated the mechanism(s) that may be involved in conferring the difference in postinfection NF-κB induction profile exhibited by HPIV-3 (rapid induction) and RSV (late induction). Recently TLRs have been shown to be used by RNA cytoplasmic viruses for rapid activation of NF-κB in infected cells (5, 6, 8). Because MyD88 is an essential TLR adaptor protein required for optimal TLR-dependent NF-κB activation (33, 34), we investigated the requirement of MyD88 in transducing RSV- and HPIV-3-mediated NF-κB activation signal.
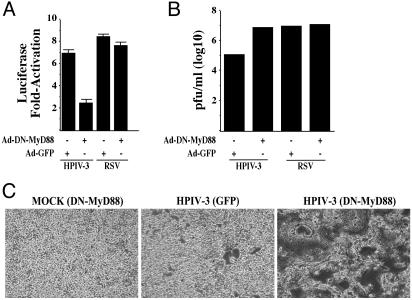
Whereas infection of A549 cells with Ad-DN-MyD88 abrogated NF-κB-Luc activation by HPIV-3, Ad-DN-MyD88 failed to inhibit NF-κB induction by RSV (Fig. 5A). Moreover, the requirement of MyD88 in HPIV-3-mediated induction of NF-κB was borne out by the observation that expression of DN-MyD88 resulted in drastic increase in HPIV-3 replication and cytopathogenicity (Fig. 5 B and C), which was similar to that observed after expression of IκB-SR (Fig. 2 B and E). These results indicate that in human lung epithelial cells, HPIV-3 and RSV uses two alternative mechanisms, MyD88-dependent and -independent pathways, respectively, to induce NF-κB. Moreover, MyD88-dependent and -independent pathways adopted by these two viruses to either induce NF-κB rapidly (HPIV-3) or late after replication initiation (RSV), respectively, may have a direct bearing on their respective cytopathogenic phenotype.
Fig. 5.
Differential requirement of MyD88 for NF-κB induction by HPIV-3 and RSV. (A) Expression of transfected NF-κB-Luc in A549 cells infected with HPIV-3 or RSV in the presence of DN-MyD88 or control GFP. (B) The indicated medium supernatants from A549 cells infected with HPIV-3 or RSV in the absence or presence of prior infection with Ads encoding the DN-MyD88 or GFP were subjected to plaque assay analysis. (C) Phase contrast microscopic picture of CV-1 cells incubated with same dilutions of medium supernatants obtained from A549 cells infected with HPIV-3 and Ad-GFP or Ad-DN-MyD88.
Discussion
In this article, we have established that two medically important human respiratory tract pathogens, HPIV-3 and RSV, respond differentially to innate response elicited by NF-κB. The mildly cytopathic virus, HPIV-3 (which induces NF-κB early during infection), was converted to a virulent virus as a result of increased replication after inhibition of rapid NF-κB induction by HPIV-3. In contrast, the replication of RSV (which induces NF-κB late in infection) was not altered in NF-κB-inhibited cells. However, specific induction of NF-κB by TNF-α or IL-1β before virus replication rendered an antiviral state against RSV, converting this highly cytopathic virus to a less virulent virus. Similar results with drastic inhibition of viral replication after induction of NF-κB before virus infection were obtained when another highly cytopathic RNA cytoplasmic virus, VSV, which possesses similar high titer like RSV in A549 cells (data not shown), and fails to induce NF-κB in these cells (21) was allowed to infect TNF-α- and IL-1β-pretreated cells (see Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). Moreover, similar to RSV, anti-VSV activity of TNF-α and IL-1β was elicited specifically by NF-κB, which is independent of IFN and soluble secreted factors (see Fig. 8, which is published as supporting information on the PNAS web site). These results demonstrated that NF-κB is also capable of conferring an essential IFN-independent antiviral activity against a virus that does not induce its activity during natural infection.
It is interesting to note that although the antiviral potential of TNF-α has been reported earlier (35, 36), the mechanism(s) involved in conferring the antiviral response was not known. In our current study, we have demonstrated that the antiviral activity of TNF-α is mediated directly by NF-κB, which is independent of IFN. In addition, we have demonstrated the ability of IL-1β to act as a potent antiviral cytokine, which exerts its IFN-independent antiviral activity by inducing NF-κB. The ability of two NF-κB-inducing cytokines to severely restrict virus replication similar to IFN demonstrated the importance of NF-κB in antiviral defense. Moreover, the noninvolvement of IFN in exerting a NF-κB-dependent antiviral response was borne out by previous reports that infection of STAT-1–/– cells yielded similar HPIV-3 titer compared with the WT cells (13), and RSV is insensitive to the antiviral action of IFN-α/β in A549 cells (37). Our results demonstrating that TNF-α and IL-1β exert their NF-κB-dependent antiviral action independent of IFN were recently validated, because microarray analysis did not reveal induction of IFN-α/β genes after treatment of cells with either TNF-α or IL-1β (38). In addition, nitric oxide (NO) production after inducible nitric oxide synthase (iNOS) induction through NF-κB (39) was not the antiviral factor, because A549 cells treated with the iNOS-competitive inhibitor l-NMMA during HPIV-3 infection resulted in no significant differences in HPIV-3 virus titer (data not shown). Moreover, the NF-κB-dependent antiviral action is not mediated by IFN-γ, because the nonimmune cells used in our studies are incapable of inducing IFN-γ gene.
Because NF-κB-mediated antiviral response critically relies on the time frame of its activation after virus infection, we further demonstrated that at least for RSV and HPIV-3, the temporal nature of NF-κB induction appears to depend on the use of MyD88. (33, 34). HPIV-3 used the MyD88-dependent pathway to rapidly induce NF-κB, probably after interaction of HPIV-3 envelope protein(s) with cell-surface TLRs during virus entry. In support of rapid activation of NF-κB by HPIV-3, a previous study (26) has reported the induction of MHC-I (a gene whose expression is regulated by transactivating function of NF-κB) by UV-irradiated (replication incompetent) HPIV-3 and UV-inactivated HPIV-3-induced NF-κB in A549 cells (data not shown). Further studies will be needed to identify the specific TLRs involved in NF-κB activation by HPIV-3. In contrast to HPIV-3, RSV induced NF-κB late after replication initiation via the MyD88-independent pathway. In that context, previous studies have shown that in contrast to alveolar macrophages or monocytes, RSV activated NF-κB late during infection in lung epithelial cells (21, 22). Moreover, the NF-κB activation by RSV in these cells were replication dependent, because UV-inactivated RSV (data not shown and ref. 40), and virus replication inhibitors (22) failed to activate RSV-dependent NF-κB induction in A549 cells. Similar to our observation, a recent study (41) has also reported TLR4-independent and replication-dependent activation of NF-κB by RSV in lung epithelial cells. In addition to MyD88, we observed a differential requirement of phosphatidylinositol 3-kinase (PI3K) (42) for induction of NF-κB by HPIV-3 and RSV. Whereas RSV failed to induce NF-κB in A549 cells after inhibition of PI3K activity (ref. 43 and data not shown), such inhibition had no effect on HPIV-3-mediated NF-κB activation (data not shown).
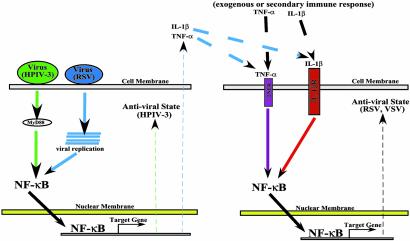
Based on our results, we propose a model for the direct establishment of an IFN-independent innate antiviral state after NF-κB activation (Fig. 6). Infection of human epithelial cells with HPIV-3 rapidly induces (replication independent) NF-κB via the MyD88-dependent IKK/IκB pathway, leading to the establishment of an antiviral state. HPIV-3, a mildly cytopathic, TNF-α-nonproducing virus (25, 26), thus induces the NF-κB antiviral pathway rapidly to restrict its own replication in infected cells. In contrast, NF-κB failed to exert its antiviral function against viruses, like RSV, which activated NF-κB via a MyD88-independent and replication-dependent pathway. Although RSV and VSV circumvents the antiviral activity of NF-κB in infected cells, activation of NF-κB by TNF-α and IL-1β (proinflammatory cytokines whose gene is regulated by transactivating function of NF-κB) before virus replication established an intracellular antiviral state. Thus, in the absence of IFN sensitivity (37) and evasion of NF-κB-dependent antiviral response, TNF-α and/or IL-1β produced by RSV-infected cells (44) may prime uninfected cells by means of binding to their cognate receptors, to restrict the spread of RSV by activating NF-κB. Moreover, TNF-α and/or IL-1β produced after secondary adaptive response by immune cells (45, 46), could prime uninfected cells to create an NF-κB-dependent antiviral state against viruses such as VSV, that do not induce NF-κB (21), and fail to produce IFN from infected cells (47).
Fig. 6.
A model depicting NF-κB-mediated innate antiviral response independent of IFN. Rapid activation of NF-κB by viruses like HPIV-3 via the MyD88 pathway early during infection in a replication-independent manner confers an intracellular antiviral state in the infected cells (Left). Similarly, viruses like RSV that induce NF-κB late after infection in a replication-dependent manner, produce TNF-α and/or IL-1β, which, by means of the paracrine mechanism, may prime uninfected cells (Right) after binding to its cognate receptors to establish an NF-κB-dependent antiviral state. In addition, exogenously added TNF-α or IL-1β, and the production of these cytokines after secondary adaptive response by immune cells, could prime uninfected cells to activate NF-κB-mediated antiviral response against viruses like VSV that do not induce NF-κB. TNFR, TNF-α receptor; IL-1βR, IL-1β receptor.
In conclusion, we report an innate antiviral immune response that is independent of the well established IFN-induced JAK/STAT pathway, and demonstrate that this innate antiviral response is directly mediated by NF-κB after its activation, either by a virus or by proinflammatory cytokines like TNF-α and IL-1β. Thus, NF-κB acts as an essential host antivirulence factor to restrict the systemic spread of pathogens by not only producing IFN-β but also by being capable of directly establishing an IFN-independent intracellular antiviral state against several RNA cytoplasmic viruses by an alternative pathway.
Supplementary Material
Acknowledgments
We thank Dr. V. M. Dixit and Dr. A. Deb for the DN-MyD88 and Ad constructs; Dr. M. Karin for the gift of IKKγ knockout MEFs; Dr. R. H. Silverman for assistance with the manuscript; Dr. K. Panda for performing the iNOS assay; Dr. George R. Stark and Dr. Andrew Lerner for the STAT null cells; and The Cleveland Clinic Foundation Virus Core Facility for providing the viruses. This work was supported by a fellowship from the Morgenthaler Foundation (to S.B.) and by grants from the National Institutes of Health (to A.K.B. and J.A.D.) and the U.S. Department of Defense (to J.A.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HPIV-3, human parainfluenza virus type 3; RSV, human respiratory syncytial virus; VSV, vesicular stomatitis virus; TNF-α, tumor necrosis factor α; JAK, Janus kinase; STAT, signal transducers and activators of transcription; MEF, mouse embryonic fibroblast; PDTC, pyrollidine dithiocarbamate; moi, multiplicity of infection; IκB-SR, IκB super repressor; Ad, adenovirus; l-NMMA, NG-monomethyl-l-arginine; CM, conditioned medium; TLR, Toll-like receptor; DN-MyD88, dominant negative TLR adaptor protein MyD88; NF-κB-Luc, NF-κB-luciferase.
References
- 1.Baldwin, A. S. (2001) J. Clin. Invest. 107, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh, S. & Karin, M. (2002) Cell 109, S81–S96. [DOI] [PubMed] [Google Scholar]
- 3.Li, X. & Stark, G. R. (2002) Exp. Hematol. (Charlottesville, Va) 30, 285–296. [DOI] [PubMed] [Google Scholar]
- 4.Li, Q. & Verma, I. M. (2002) Nat. Rev. 2, 725–734. [DOI] [PubMed] [Google Scholar]
- 5.Bieback, K., Lien, E., Klagge, I. M., Avota, E., Schneider-Schaulies, J., Duprex, W. P., Wagner, H., Kirschning, C. J., Ter Meulen, V. & Schneider-Schaulies, S. (2002) J. Virol. 76, 8729–8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurt-Jones, E. A., Popova, L., Kwinn, L., Haynes, L. M., Jones, L. P., Tripp, R. A., Walsh, E. E., Freeman, M. W., Golenbock, D. T., Anderson, L. J. & Finberg, R. W. (2000) Nat. Immunol. 1, 398–401. [DOI] [PubMed] [Google Scholar]
- 7.Mogensen, T. H. & Paludan, S. R. (2001) Microbiol. Mol. Biol. Rev. 65, 131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helin, E., Vainionpaa, R., Hyppia, T., Julkunen, I. & Matikainen, S. (2001) Virology 290, 1–10. [DOI] [PubMed] [Google Scholar]
- 9.Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. (1998) Annu. Rev. Biochem. 67, 227–264. [DOI] [PubMed] [Google Scholar]
- 10.Bose, S., Malur, A. & Banerjee, A. K. (2001) J. Virol. 75, 1984–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb, R. A. & Kolakofsky, D. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott, Williams & Wilkins, Philadelphia), pp. 1305–1340.
- 12.Makris, C., Godfrey, V. L., Krahn-Senftlenben, G., Takahashi, T., Roberts, J. L., Schwarz, T., Feng, L., Johnson, R. S. & Karin, M. (2000) Mol. Cell 5, 969–979. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary, S., Gao, J., Leaman, D. W. & De, B. P. (2001) J. Virol. 75, 4823–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian, B., Zhang, Y., Luxon, B. A., Garofalo, R. P., Casola, A., Sinha, M. & Brasier, A. R. (2002) J. Virol. 76, 6800–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose, S. & Banerjee, A. K. (2002) Virology 298, 73–83. [DOI] [PubMed] [Google Scholar]
- 16.Elewaut, D., DiDonato, J. A., Kim, J. M., Truong, F., Eckmann, L. & Kagnoff, M. F. (1999) J. Immunol. 163, 1457–1466. [PubMed] [Google Scholar]
- 17.DiDonato, J. A., Mercurio, F., Rosette, C., Wu-Li, J., Suyang, H., Ghosh, S. & Karin, M. (1996) Mol. Cell. Biol. 16, 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez, A. & Banerjee, A. K. (1985) Virology 143, 45–53. [DOI] [PubMed] [Google Scholar]
- 19.Gupta, S., De, B. P., Drazba, J. A. & Banerjee, A. K. (1998) J. Virol. 72, 2655–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broughan, J. H., Randolph, V. B. & Tatem, J. M. (1997) J. Virol. 71, 4962–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitko, V., Velazquez, A., Yang, L., Yu-Chung., Y. & Barik, S. (1997) Virology 232, 369–378. [DOI] [PubMed] [Google Scholar]
- 22.Fiedler, M. A., Wernke-Dollries, K. & Stark, J. M. (1996) J. Virol. 70, 9079–9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan, P. & O'Neill, L. A. (1996) Biochem. J. 320, 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, G. Y., Le, S., Park, K. H., Le, C. T., Kim, Y. M., Han, S. K., Shim, Y. S. & Yoo, C. G. (2001) Eur. Respir. J. 18, 801–809. [DOI] [PubMed] [Google Scholar]
- 25.Gao, J., Choudhary, S., Banerjee, A. K. & De, B. P. (2000) Gene Expression 9, 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao, J., De, B. P. & Banerjee, A. K. (1999) J. Virol. 73, 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallach, D., Varfolomeev, E. E., Malinin, N. L., Goltsev, Y. V., Kovalenko, A. V. & Boldin, M. P. (1999) Annu. Rev. Immunol. 17, 331–367. [DOI] [PubMed] [Google Scholar]
- 28.Auron, P. E. (1998) Cytokine Growth Factor Rev. 9, 221–237. [DOI] [PubMed] [Google Scholar]
- 29.Kyriakis, J. M. & Avruch, J. (1996) BioEssays 18, 567–577. [DOI] [PubMed] [Google Scholar]
- 30.Li, X., Commane, M., Jiang, Z. & Stark, G. R. (2001) Proc. Natl. Acad. Sci. USA 98, 4461–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKendry, R., John, J., Flavell, D., Muller, M., Kerr, I. M. & Stark, G. R. (1999) Proc. Natl. Acad. Sci. USA 88, 11455–11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhopadhyay, A., Shishodia, S., Fu, X. Y. & Aggarwal, B. B. (2002) J. Cell. Biochem. 84, 803–815. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald, K. A., Palsson-McDermott, E. M., Bowie, A. G., Jefferies, C. A., Mansell, A. S., Brady, G., Brint, E., Dunne, A., Gray, P., Harte, M. T., et al. (2001) Nature 413, 78–83. [DOI] [PubMed] [Google Scholar]
- 34.Muzio, M., Ni, J., Feng, P. & Dixit, V. M. (1997) Science 278, 1612–1615. [DOI] [PubMed] [Google Scholar]
- 35.Merolla, R., Rebert, N. A., Tsiviste, P. T., Hoffmann, S. P. & Panuska, J. R. (1995) Am. J. Respir. Crit. Care Med. 152, 1358–1366. [DOI] [PubMed] [Google Scholar]
- 36.Neuzil, K. M., Tang, Y. & Graham, B. S. (1996) Am. J. Med. Sci. 311, 201–204. [DOI] [PubMed] [Google Scholar]
- 37.Atreya, P. L. & Kulkarni, S. (1999) Virology 261, 227–241. [DOI] [PubMed] [Google Scholar]
- 38.Li, X., Massa, P. E., Hanidu, A., Peet, G. W., Aro, P., Savitt, A., Mische, S., Li, J. & Marcu, K. B. (2002) J. Biol. Chem. 277, 45129–45140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asano, K., Chee, C., Gaston, B., Lilly, C. M., Gerard, C., Drazen, J. M. & Stamler, J. S. (1994) Proc. Natl. Acad. Sci. USA 91, 10089–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garofalo, R., Sabry, M., Jamaluddin, M., Yu, R., Casola, A., Ogra, P. L. & Brasier, A. R. (1996) J. Virol. 70, 8773–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haeberle, H. A., Takizawa, R., Casola, A., Brasier, A. R., Dieterich, H. J., Van-Rooijen, N., Gatalica, Z. & Garofalo, R. P. (2002) J. Infect. Dis. 186, 1199–1206. [DOI] [PubMed] [Google Scholar]
- 42.Sizemore, N., Leung, S. & Stark, G. R. (1999) Mol. Cell. Biol. 19, 4798–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas, K. W., Monick, M. M., Staber, J. M., Yarovinsky, T., Carter, A. B. & Hunninghake, G. W. (2002) J. Biol. Chem. 277, 492–501. [DOI] [PubMed] [Google Scholar]
- 44.Tsutsumi, H., Takeeuchi, R., Ohsaki, M., Seki, K. & Chiba, S. (1999) J. Leukocyte Biol. 66, 99–104. [PubMed] [Google Scholar]
- 45.Bluman, E. M., Bartynski, K. J., Avalos, B. R. & Caligiuri, M. A. (1996) J. Clin. Invest. 97, 2722–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vreugdenhil, G. R. (2000) Cytokine 12, 1793–1796. [DOI] [PubMed] [Google Scholar]
- 47.Ferran, M. C. & Lucas-Lenard, J. M. (1997) J. Virol. 71, 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.