Abstract
A variety of tumor-derived antigens have been defined by IgG antibodies in tumor bearers' sera with serological identification of antigens by recombinant expression cloning (SEREX), a serological expression cloning method. The majority of these antigens show no structural abnormality and seem to be wild-type autoantigens. Coimmunization with DNA encoding these autoantigens and tumor-specific cytotoxic T lymphocytes epitopes heightened CD8+ T cell responses and increased resistance to tumor challenge in a CD4+ T cell-dependent manner. In contrast, immunization with these SEREX-defined autoantigens alone leads to heightened susceptibility to tumor challenge. This suppressive effect of immunization is mediated by CD4+ CD25+ T cells. In mice immunized with one of the SEREX-defined autoantigens, Dna J-like 2, the number of α-GalCer/CD1d tetramer+ CD3+ T cells [representing natural killer T (NKT) cells] was reduced in the pulmonary compartment, whereas no evident change in the number of other T cell subsets was observed. Experiments with Jα281–/– mice lacking most NKT cells indicate that NKT cells are primarily responsible for metastasis suppression and that their activity is inhibited by immunization with Dna J-like 2. We propose that SEREX identifies a pool of autoantigens that maintains and regulates immunological homeostasis via CD4+ CD25+ regulatory T cells.
Antitumor immune responses involve complex interactions among various immunocompetent cells. CD8+ cytotoxic T lymphocytes (CTLs) are major effector cells capable of direct tumor destruction both in vivo and in vitro, and they recognize peptide targets with strict MHC class I restriction (1–3). Natural killer T (NKT) cells represent critical effector cells in innate type immune response (4–10). NKT cells possess a T cell receptor of extremely limited diversity, using the Vα14-Jα281 rearrangement in mice and Vα24-JαQ in humans (4, 8, 10). NKT T cell antigen receptor binds CD1d complexed with the glycolipid, α-galactosylceramide (α-GalCer), which is not present in mammalian hosts, but presumably mimics the action of naturally derived glycolipids (6).
Whereas CD4+ T cells do not generally destroy tumor target cells directly, they play important roles in regulating antitumor immune responses through various mechanisms. CD4+ helper T cells recognizing cognate tumor antigen peptides presented by MHC class II molecules on antigen-presenting cells amplify the activation and clonal expansion of CD8+ T cells (11–15). Regulatory T cells having CD4/CD25 phenotypes are also of considerable interest (16–21). They have been shown to suppress the development of autoimmunity (16–20) and transplantation immunity (21, 22) and more recently to be involved in antitumor immunity (23, 24). Although these regulatory T cells are thought to recognize self-antigen peptides (25, 26), the molecular profiles of the antigens they respond to are unknown. Despite the growing recognition of the importance of CD4+ CD25+ T cells, the antigenic and functional relationships between regulatory and helper T cells remain elusive.
We recently reported that immunization with immunogenic wild-type molecules derived from chemically induced murine sarcomas strongly augments the response of CD8+ T cells specific for tumor rejection antigens (27). These immunogenic molecules were identified by an expression cloning method, serological identification of antigens by recombinant expression cloning (SEREX), using IgG class antibodies present in sera obtained from tumor-bearing hosts (27–31). Coimmunization of mice with a mixture of plasmids encoding CD8+ T cell recognizing tumor antigen and SEREX-defined antigens remarkably increase the number of tumor-specific CD8+ CTLs and heighten resistance to challenges with the cognate tumor. CD4+ helper T cells were found to be critical in mediating these antitumor immune responses (27).
In the present study, we report the surprising finding that immunization with plasmids encoding these SEREX-defined wild-type antigens alone resulted in a marked enhancement of in vivo tumor growth. CD4+ CD25+ T cells were found to be responsible for this tumor enhancement.
Materials and Methods
Mice. Female BALB/c mice were obtained from CLEA Japan (Osaka) and used at 7–10 weeks of age. Vα14NKT cell-deficient (Jα281–/–) mice of BALB/c background were established by specific deletion of the Jα281 gene segment as described (5) and used at 7–9 weeks of age. Mice were maintained at the Animal Center of Mie University School of Medicine. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Mie University School of Medicine.
Tumors. CMS2, CMS5, CMS7, CMS8, and CMS13 are 3-methylcholanthrene-induced sarcoma cell lines of BALB/c origin (32). CMS5a and CMS5m are subcloned cell lines obtained from CMS5, a tumor expressing mutated ERK2 (mERK2) (27, 33). Colon 26 is a colorectal adenocarcinoma cell line of BALB/c origin (34).
Plasmids. SEREX-defined molecules and control SEREX-unrelated molecules (27) were cloned into pBK-CMV (Stratagene) and purified by using Qiagen EndoFree Plasmid Mega kit (Qiagen, Hilden, Germany).
Immunization by Gene Gun. Seven-week-old BALB/c mice received abdominal wall delivery of plasmid DNA-coated gold particles by using a Helios Gene Gun system (Bio-Rad) at a helium discharge pressure of 350–400 psi as described (27). Gold particles were prepared as described (27).
Analysis of Pulmonary Metastasis. BALB/c mice were challenged with 1 × 106 CMS5m or 1 × 105 Colon 26 tumor cells in a total volume of 0.1 ml through the lateral tail vein. Twenty-eight (CMS5m) or 21 (Colon 26) days later, mice were killed, and the number of pulmonary nodules was counted with a dissecting microscope as described (27).
Antibodies and Reagents. Anti-CD25 (PC61, rat IgG1) was purified from ascites on a DEAE Toyopearl 650S column (Tosoh, Tokyo). The mAbs specific for CD4 (GK1.5, rat IgG2b) and CD8 (19/178, rat IgG2b) were used in the form of ascites. α-GalCer was kindly provided by Pharmaceutical Research Laboratories (Kirin Brewery, Gunma, Japan) (6).
Transfer of CD4+ CD25+ and CD4+ CD25– T Cells. Spleen cells were fractionated into CD25+ and CD25– by using MACS anti-FITC MultiSort kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Anti-FITC microbeads bound to CD25+ cells were detached by using MACS MultiSort Release Reagent and MACS MultiSort Stop Reagent according to the instructions provided by the manufacturer. The resultant CD25+ and CD25– cells were further enriched for CD4+ cells by positive selection on a MACS column after reacting with anti-CD4 microbeads. CD4+ CD25+ and CD4+ CD25– T cell preparations were confirmed to contain >95% CD4+ CD25+ T cells and >92% CD4+ CD25– T cells, respectively. Purified CD4+ CD25+ T cells or CD4+ CD25– T cells were inoculated into BALB/c mice through the lateral tail vein.
Analysis of Lung Mononuclear Cells. Lung mononuclear cells were isolated as described (35). Briefly, the lung was cut into small pieces followed by incubation for 30 min at 37°C in RPMI medium 1640 containing 0.05% collagenase and 0.01% trypsin inhibitor. The resultant single cell suspensions were passed through a PreSeparation Filter (Miltenyi Biotec), and subsequently centrifuged over 33% Percoll. The mononuclear cells were collected for FACS analysis after removal of red blood cells. Cells were incubated with PerCP-anti-CD3 (145–2C11) (PharMingen) and PE-α-GalCer/CD1d tetramer in PBS containing 2% FCS and 0.02% NaN3 for 20 min at 4°C. To prevent nonspecific staining, cells were pretreated with anti-FcγR II/III mAb (2.4G2) for 15 min. The stained cells were subjected to two-color analysis on FACScalibur (Becton Dickinson). α-GalCer/CD1d tetramer was prepared as described (36).
Reconstitution of Jα281–/– Mice with α-GalCer/CD1d Tetramer+ Cells. Splenocytes from BALB/c mice were enriched for α-GalCer/CD1d tetramer+ cells by using anti-PE microbeads (Miltenyi Biotec), as described (36) with some modifications. Briefly, splenocytes were pretreated with anti-FcγR II/III mAb (2.4G2) for 15 min and incubated with α-GalCer/CD1d tetramer followed by anti-PE microbeads. α-GalCer/CD1d tetramer+ cells were isolated by positive selection on a MACS column, and 2 × 105 α-GalCer/CD1d tetramer+ cells were transferred to Jα281–/– mice by injection through the lateral tail vein.
Results
Enhancement of Pulmonary Metastasis in Hosts Immunized with SEREX-Defined Wild-Type Antigens. Injection (i.v.) of syngeneic sarcoma BALB/c CMS5m, a 3-methylcholanthrene-induced tumor expressing mERK2, resulted in the appearance of pulmonary metastasis followed by death within 5–6 weeks (27). As we reported (27), immunization of mice with a combination of plasmids encoding mERK2 with a CTL epitope, 9m (33), and SEREX-defined wild-type antigens completely prevented lung metastasis when initiated 5 days after challenge with CMS5m.
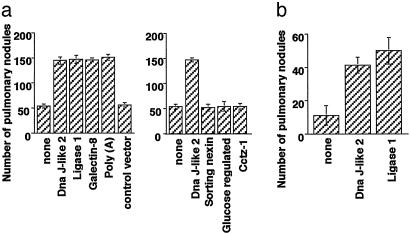
To investigate the effect of immunization with SEREX-defined wild-type antigen alone, mice were immunized 5 days after tumor challenge with plasmids encoding the SEREX-defined wild-type antigens or with control plasmids. Mice were killed 28 days after CMS5m injection, and the number of pulmonary metastatic nodules was counted. The number of pulmonary nodules in mice immunized with plasmids encoding any one of four different SEREX-defined wild-type antigens, Mus heat shock protein, Dna J-like 2 (Dna J-like 2, GenBank accession no. AF055664), Mus DNA ligase 1 (Ligase 1, GenBank accession no. U19604), Mus galectin-8 (Galectin-8, GenBank accession no. AF218069), and Mus poly(A)-binding protein, cytoplasmic 1 [poly(A), GenBank accession no. X65553], ranged from 130 to 170, whereas the number in control mice was 30–60 nodules (Fig. 1a). In contrast, no increase in the number of metastatic nodules was observed in mice immunized with control plasmids or with plasmids encoding murine molecules not detected in repeated SEREX analyses and selected randomly from SEREX screening library, Mus sorting nexin 1 (sorting nexin, GenBank accession no. AB019214), Mus glucose-regulated protein (Glucose-regulated, GenBank accession no. D78645), and Mus Cctz-1 gene for chaperon containing TCP-1-zeta-1 subunit (Cctz-1, GenBank accession no. AB022159) (Fig. 1a). A similar experiment was performed by using another BALB/c syngeneic murine tumor, Colon 26. Immunization with either of two SEREX-defined wild-type antigens, Dna J-like 2 or Ligase 1, also resulted in tumor enhancement as measured by increased number of metastatic nodules (Fig. 1b).
Fig. 1.
Immunization with SEREX-defined wild-type antigens enhances pulmonary metastasis. BALB/c mice were injected with 1 × 106 CMS5m (a) or 1 × 105 Colon 26 (b) via the lateral tail vein. Biweekly immunization with plasmids encoding the indicated antigens by using a gene gun commenced 5 days after tumor challenge. Pulmonary metastatic nodules were counted with a dissecting microscope 28 (a) or 21 (b) days after tumor challenge. Data are mean ± SD; n = 5. These experiments were repeated three times with similar results.
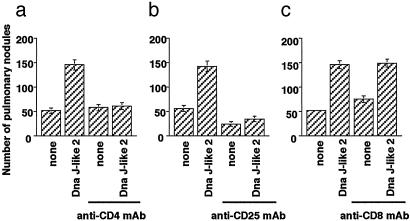
Enhancement of Pulmonary Metastasis Depends on CD4+ CD25+ T Cells. We then analyzed the cell phenotypes involved in enhancement of pulmonary metastasis. Pretreatment of mice with anti-CD4 mAb (GK1.5) or anti-CD25 mAb (PC61) suppressed the tumor enhancement induced by immunization with SEREX-defined wild-type antigen, Dna J-like 2 (Fig. 2 a and b). Nonimmunized mice pretreated with anti-CD25 mAb alone had significantly fewer metastatic nodules compared with nonpretreated mice (Fig. 2b). In contrast, Dna J-like 2 immunized mice pretreated with anti-CD8 mAb (19/178) showed no change in the number of pulmonary metastatic nodules (Fig. 2c). The number of metastatic nodules was slightly increased in nonimmunized mice pretreated with anti-CD8 mAb (Fig. 2c).
Fig. 2.
CD4+ and CD25+ cells but not CD8+ cells are responsible for enhancement of pulmonary metastasis induced by immunization with SEREX-defined wild-type antigen. BALB/c mice were injected i.p. with anti-CD4 mAb (25-μl ascites, a), anti-CD25 mAb (250 μg, b), or anti-CD8 mAb (25-μl ascites, c) 2 days before tumor challenge. Mice treated with anti-CD4 mAb and anti-CD8 mAb injection were also treated with the respective mAb every 7 days. Mice were injected with 1 × 106 CMS5m cells intravenously. Biweekly immunization with plasmids encoding Dna J-like 2 commenced 5 days after tumor challenge. Pulmonary metastatic nodules were counted with a dissecting microscope 28 days after tumor challenge. Data are mean ± SD; n = 5. These experiments were repeated three times with similar results.
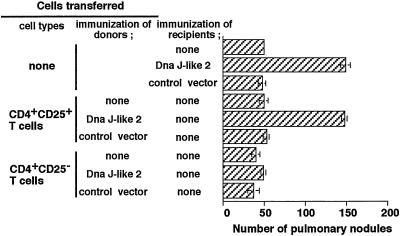
Adoptive Transfer of CD4+ CD25+ T Cells Enhances Pulmonary Metastasis. To analyze the role of CD4+ CD25+ T cells in tumor enhancement, we asked whether CD4+ CD25+ T cells derived from Dna J-like 2 immunized animals could enhance pulmonary metastasis in recipient mice. As shown in Fig. 3, CD4+ CD25+ T cells, but not CD4+ CD25– T cells derived from animals immunized with Dna J-like 2, markedly enhanced pulmonary metastasis in recipients. No enhancement of metastasis was observed in recipients of CD4+ CD25+ T cells derived from naive animals or animals immunized with control vector (Fig. 3).
Fig. 3.
Enhancement of pulmonary metastasis by transfer of CD4+ CD25+ T cells from mice immunized with SEREX-defined wild-type antigen. Purified 1 × 105 CD4+ CD25+ T cells or CD4+ CD25– T cells derived from BALB/c mice (naive, Dna J-like 2, or control vector immunized) were transferred into naive BALB/c mice 7 days before 1 × 106 CMS5m inoculation. Mice were killed 28 days after tumor challenge, and pulmonary metastatic nodules were counted with a dissecting microscope. Data are mean ± SD; n = 5. These experiments were repeated three times with similar results.
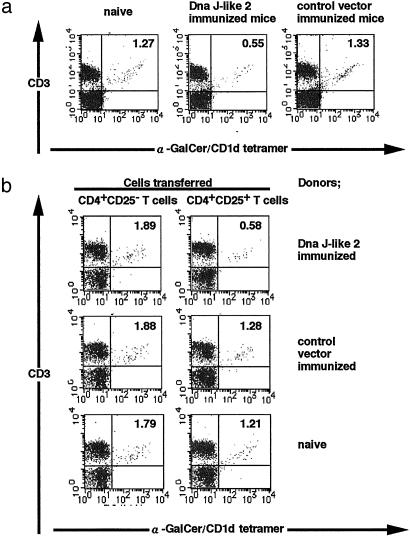
Reduction of α-GalCer/CD1d Tetramer+ Cells in Pulmonary Compartments by Regulatory T Cells. We then analyzed the influence of immunization with SEREX-defined wild-type antigens on T cell types in the lungs. Immunization with SEREX-defined wild-type antigens resulted in a significant decrease in the proportion of α-GalCer/CD1d tetramer+ cells as well as their total number in the lungs (Fig. 4a). In contrast, there were no significant changes in the absolute and relative numbers of CD4+ T cells (both CD4+ CD25+ and CD4+ CD25– T cells) or CD8+ T cells (data not shown).
Fig. 4.
Reduction of α-GalCer/CD1d tetramer+ cells in lungs of mice immunized with Dna J-like 2 or of mice injected with CD4+ CD25+ T cells from Dna J-like 2 immunized mice. Pulmonary mononuclear cells from BALB/c mice (naive or immunized with Dna J-like 2 or control vector) (a) or transferred with CD4+ CD25+ T cells or CD4+ CD25– T cells (naive or immunized with Dna J-like 2 or control vector) were stained with PE-α-GalCer/CD1d tetramer and PerCP-anti-CD3. These cells were subjected to two-color analysis on FACSCalibur. The annotated numbers indicate the percentage of cells in each quadrant. These experiments were repeated three times with similar results.
A similar analysis was performed in recipients of CD4+ T cells from Dna J-like 2 immunized mice. Reduction of α-GalCer/CD1d tetramer+ cells was also observed in the lung of recipients of CD4+ CD25+ T cells derived from animals immunized with SEREX-defined wild-type antigen, Dna J-like 2 (Fig. 4b). However, there was no significant change in other lymphoid cell populations also in these mice (data not shown). Transfer of CD4+ CD25+ T cells derived from naive animals resulted in essentially no reduction of α-GalCer/CD1d tetramer+ cells in the recipients (Fig. 4b).
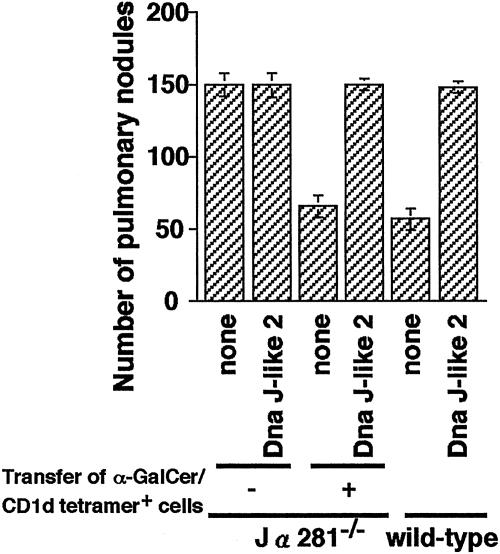
Involvement of α-GalCer/CD1d Tetramer+ Cells in the Inhibition and Enhancement of Pulmonary Metastasis. We therefore investigated the role of NKT cells in the inhibition/enhancement of pulmonary metastasis induced by immunization with SEREX-defined wild-type antigens and the possibility that NKT cells are the targets of immunosuppression by CD4+ CD25+ T cells. Jα281–/– mice, essentially lacking Vα14NKT cells while having the rest of their immune system intact (5), showed increased number of pulmonary metastasis as compared with wild-type mice. We transferred α-GalCer/CD1d tetramer+ cells derived from wild-type BALB/c mice into Jα281–/– mice. Jα281–/– mice reconstituted with α-GalCer/CD1d tetramer+ cells developed the same number of metastatic nodules as wild-type mice (Fig. 5). Jα281–/– mice reconstituted with α-GalCer/CD1d tetramer+ cells followed by Dna J-like 2 immunization showed similar enhancement of pulmonary metastasis as observed in Dna J-like 2 immunized wild-type animals (Fig. 5).
Fig. 5.
Enhancement of pulmonary metastasis is caused by regulation of NKT cells. Jα281–/– mice were reconstituted with 2 × 105 α-GalCer/CD1d tetramer+ cells derived from naive wild-type mice. On the day of reconstitution, 1 × 106 CMS5m were inoculated. The mice were immunized twice with plasmids encoding Dna J-like 2 at 2-week intervals. Mice, either reconstituted with α-GalCer/CD1d tetramer+ cells or not, and also immunized with Dna J-like 2 or not, were killed 28 days after tumor inoculation, and pulmonary metastatic nodules were counted with a dissecting microscope. Data are mean ± SD; n = 5. These experiments were repeated three times with similar results.
Discussion
Recognition of SEREX-Defined Antigens by CD4+ Helper T Cells. SEREX assays have been widely used to identify immunogenic molecules in tumors of mice and humans (27–31). IgG antibodies in the sera of tumor-bearing hosts are used to screen λ-phage libraries prepared from cDNA of tumor cells, and hence the SEREX repertoire can be considered to be a reflection of CD4+ T cell repertoire. We used SEREX methodology to identify immunogenic molecules in several 3-methylcholanthrene-induced sarcoma lines of BALB/c origin. Screening cDNA expression libraries of tumors with sera of animals bearing cognate tumors identified 21 different genes. Among the array of genes detected, four of the most frequently detected gene products were used in the present study after verifying the lack of any gene mutations based on the registered sequences in GenBank. Coimmunization of mice with plasmids encoding these SEREX-defined wild-type antigens and mERK2 (containing tumor-specific CTL epitope 9m of CMS5) led to a profound increase in CD8+ T cells specific for mERK2 (27). This heightened response depends on CD4+ T cells and copresentation of SEREX-defined wild-type antigens and the CTL epitope. In tumor protection assay, coimmunization with SEREX-defined wild-type antigens and mERK2 also resulted in a striking CD4+ T cell-dependent inhibition of pulmonary metastasis of CMS5m, which could not be achieved by immunization with mERK2 alone (27). These results indicated the essential role of CD4+ T cells in mediating the increased CD8+ T cell response and tumor inhibition induced by coimmunization with SEREX-defined antigens.
CD4+ CD25+ Regulatory T Cells Induced by Immunization with SEREXDefined Wild-Type Antigens. Immunization of hosts with SEREX-defined wild-type antigens alone, on the other hand, resulted in marked enhancement of pulmonary metastasis after i.v. administered tumor cells. The essential role of CD4+ CD25+ T cells in this enhancement is evident from experiments showing that enhanced metastasis is (i) eliminated by treatment with anti-CD4 or anti-CD25 mAbs and (ii) can be induced by adoptive transfer of CD4+ CD25+ T cells from immunized mice to naive recipients. As few as 1 × 105 CD4+ CD25+ T cells derived from mice immunized with Dna J-like 2 led to profound enhancement of pulmonary metastasis, whereas CD4+ CD25+ T cells derived from naive mice induced no enhancement.
CD4+ CD25+ T cells are known to regulate various immune responses such as autoimmunity (16–20), allogeneic transplantation immunity (21, 22), and antitumor immunity (23, 24). Evidence indicates that these T cells recognize self-antigen peptides in the context of MHC class II (25, 26). Although no particular antigenic molecules have been identified, the involvement of a variety of self-antigen peptides has been proposed (17–20, 25, 26). The present results suggest that immunogenic wild-type molecules identified by SEREX methodology may represent autoantigens recognized by CD4+ CD25+ T cells and regulate their activities. This idea is supported by the fact that immunization with four independently derived SEREX-defined wild-type antigens induced CD4+ CD25+ T cell-mediated enhancement of pulmonary metastasis and that no enhancement was induced by immunization with three mouse products randomly selected from the phage screening library that were not demonstrably immunogenic (27).
Regulation of NKT Cell Activity by CD4+ CD25+ T Cells Induced by SEREX-Defined Autoantigens. In several experimental tumor systems, NKT cells display key roles in controlling tumor growth in vivo (4–9). Their suppressive effects are most evident in tumors growing in the lungs or liver, two organs in which NKT cells exist in relatively large number (5). In our experimental system of pulmonary metastasis, NKT cells seem to be primary effectors. The transfer of metastasis inhibition by α-GalCer/CD1d tetramer+ cells into Jα281–/– recipients indicates the essential role of these cells. CD8+ CTLs are also clearly involved in tumor rejection, as the number of metastatic nodules tended to increase in mice whose CD8+ T cells were eliminated by antibodies.
In hosts immunized with SEREX-defined autoantigens, a major target of CD4+ CD25+ T cells is NKT cells. This is most clearly shown by the fact that in Jα281–/– hosts transferred with α-GalCer/CD1d tetramer+ cells, immunization with Dna J-like 2 resulted in the enhancement of pulmonary metastasis. Analysis of NKT cells by use of α-GalCer/CD1d tetramer showed a significant decrease of NKT cells in response to Dna J-like 2 immunization or by transfer of CD4+ CD25+ T cells from Dna J-like 2 immunized mice. Whereas the underlying mechanism of reduction of NKT cell number is unknown, immune suppression by regulatory T cells is in fact broader than previously proposed and includes NKT cells in addition to CD4+ and CD8+ T cells (16–20, 37).
Role of SEREX-Defined Autoantigens in Antitumor Immune Responses. Our results are consistent with the growing recognition that autoantigen-reactive regulatory T cells play a significant role in the suppression of a variety of immune responses (16–24). In our studies, the role of these cells might have been artificially amplified by vaccinating hosts by gene gun with 1 μg of plasmids, likely leading to the loading of antigen-presenting cells with vast amounts of self-antigens. Nonetheless, the current analysis provides an approach to elucidating the nature and the magnitude of T cell regulation induced by self-antigens.
In tumor-bearing hosts, a range of autoantigenic molecules derived from the tumor stimulates the production of antibodies, e.g., those detected by SEREX (27–31). CD4+ T cells recognizing these autoantigens may strengthen CD8+ T cell responses under appropriate conditions, such as following copresentation of CTL epitopes as we reported (27). In the absence of CTL epitopes, activated CD4+ T cells may acquire regulatory activity rather than helper activity, resulting in a state of immunosuppression. This scenario provides clues that may be helpful in developing of cancer vaccines and indicates the fine line between tumor inhibition and tumor enhancement that may be encountered following immunization with autoantigenic tumor antigens. More importantly, it provides new ways to think about the significance of immunogenic tumor autoantigens and the massive data pool accumulated from the SEREX analysis of human cancer (see www.licr.org/SEREX.html for more information).
Acknowledgments
This work was supported by grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Abbreviations: SEREX, serological identification of antigens by recombinant expression cloning; CTL, cytotoxic T lymphocyte; mERK2, mutated ERK2; NKT, natural killer T.
References
- 1.Wagner, H., Pfizenmaier, K. & Rollinghoff, M. (1980) Adv. Cancer Res. 31, 77–124. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg, P. D. (1991) Adv. Immunol. 49, 281–355. [DOI] [PubMed] [Google Scholar]
- 3.Melief, C. J. (1992) Adv. Cancer Res. 58, 143–175. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac, A., Rivera, M. N., Park, S.-H. & Roark, J. H. (1997) Annu. Rev. Immunol. 15, 535–562. [DOI] [PubMed] [Google Scholar]
- 5.Cui, J., Shin, T., Kawano, T., Sato, H., Kondo, E., Toura, I., Kaneko, Y., Koseki, H., Kanno, M. & Taniguchi, M. (1997) Science 278, 1623–1626. [DOI] [PubMed] [Google Scholar]
- 6.Kawano, T., Cui, J., Koezuka, Y., Toura, I., Kaneko, Y., Motoki, K., Ueno, H., Nakagawa, R., Sato, H., Kondo, E., et al. (1997) Science 278, 1626–1629. [DOI] [PubMed] [Google Scholar]
- 7.Smyth, M. J., Thia, K. Y., Street, S. E., Cretney, E., Trapani, J. A., Taniguchi, M., Kawano, T., Pelikan, S. B., Crowe, N. Y. & Godfrey, D. I. (2000) J. Exp. Med. 191, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth, M. J. & Godfrey, D. I. (2000) Nat. Immunol. 1, 459–460. [DOI] [PubMed] [Google Scholar]
- 9.Crowe, N. Y., Smyth, M. J. & Godfrey, D. I. (2002) J. Exp. Med. 196, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kronenberg, M. & Gapin, L. (2002) Nat. Rev. Immunol. 2, 557–568. [DOI] [PubMed] [Google Scholar]
- 11.Ossendorp, F., Mengedé, E., Camps, M., Filius, R. & Melief, C. J. (1998) J. Exp. Med. 187, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung, K., Hayashi, R., Lafond-Walker, A., Lowenstein, C., Pardoll, D. & Levitsky, H. (1998) J. Exp. Med. 188, 2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll, D. M. & Topalian, S. L. (1998) Curr. Opin. Immunol. 10, 588–594. [DOI] [PubMed] [Google Scholar]
- 14.Wang, R. F. (2001) Trends. Immunol. 22, 269–276. [DOI] [PubMed] [Google Scholar]
- 15.Malek, T. R. (2002) Trends Immunol. 23, 465–467. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151–1164. [PubMed] [Google Scholar]
- 17.Sakaguchi, S. (2000) Cell 101, 455–458. [DOI] [PubMed] [Google Scholar]
- 18.Shevach, E. M. (2000) Annu. Rev. Immunol. 18, 423–449. [DOI] [PubMed] [Google Scholar]
- 19.Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389–400. [DOI] [PubMed] [Google Scholar]
- 20.Maloy, K. J. & Powrie, F. (2001) Nat. Immunol. 2, 816–822. [DOI] [PubMed] [Google Scholar]
- 21.Karim, M., Bushell, A. R. & Wood, K. J. (2002) Curr. Opin. Immunol. 14, 584–591. [DOI] [PubMed] [Google Scholar]
- 22.Hara, M., Kingsley, C. I., Niimi, M., Read, S., Turvey, S. E., Bushell, A. R., Morris, P. J., Powrie, F. & Wood, K. J. (2001) J. Immunol. 166, 3789–3796. [DOI] [PubMed] [Google Scholar]
- 23.Onizuka, S., Tawara, I., Shimizu, J., Sakaguchi, S., Fujita, T. & Nakayama, E. (1999) Cancer Res. 59, 3128–3133. [PubMed] [Google Scholar]
- 24.Shimizu, J., Yamazaki, S. & Sakaguchi, S. (1999) J. Immunol. 163, 5211–5218. [PubMed] [Google Scholar]
- 25.Bensinger, S. J., Bandeira, A., Jordan, M. S., Caton, A. J. & Laufer, T. M. (2001) J. Exp. Med. 194, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan, M. S., Boesteanu, A., Reed, A. J., Petrone, A. L., Holenbeck, A. E., Lerman, M. A., Naji, A. & Caton, A. J. (2001) Nat. Immunol. 2, 301–306. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa, H., Tanida, K., Ikeda, H., Sakakura, M., Miyahara, Y., Aota, T., Mukai, K., Watanabe, M., Kuribayashi, K., Old, L. J., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 14571–14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin, U., Tureci, O., Schmitt, H., Cochlovius, B., Johannes, T., Schmits, R., Stenner, F., Luo, G., Schobert, I. & Pfreundschuh, M. (1995) Proc. Natl. Acad. Sci. USA 92, 11810–11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahin, U., Tureci, O. & Pfreundschuh, M. (1997) Curr. Opin. Immunol. 9, 709–716. [DOI] [PubMed] [Google Scholar]
- 30.Old, L. J. & Chen, Y.-T. (1998) J. Exp. Med. 187, 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, Y.-T., Scanlan, M. J., Obata, Y. & Old, L. J. (2000) in Principles and Practice of the Biologic Therapy of Cancer, ed. Rosenberg, S. A. (Lippincott, Philadelphia), pp. 557–570.
- 32.DeLeo, A. B., Shiku, H., Takahashi, T., John, M. & Old, L. J. (1977) J. Exp. Med. 146, 720–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda, H., Ohta, N., Furukawa, K., Miyazaki, H., Wang, L., Kuribayashi, K., Old, L. J. & Shiku, H. (1997) Proc. Natl. Acad. Sci. USA 94, 6375–6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corbett, T. H., Griswold, D. P., Jr., Roberts, B. J., Peckham, J. C. & Schabel, F. M., Jr. (1975) Cancer Res. 35, 2434–2439. [PubMed] [Google Scholar]
- 35.Watanabe, T., Kawamura, T., Kawamura, H., Haga, M., Shirai, K., Watanabe, H., Eguchi, S. & Abo, T. (1997) J. Immunol. 158, 5805–5814. [PubMed] [Google Scholar]
- 36.Matsuda, J. L., Naidenko, O. V., Gapin, L., Nakayama, T., Taniguchi, M., Wang, C. R., Koezuka, Y. & Kronenberg, M. (2000) J. Exp. Med. 192, 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piccirillo, C. A. & Shevach, E. M. (2001) J. Immunol. 167, 1137–1140. [DOI] [PubMed] [Google Scholar]