Abstract
Only a small number of T cells generated in the thymus each day are selected to replenish the peripheral T cell pool. Much is known about thymic selection; however, little is known of the mechanisms regulating medullary maturation and the release of mature T cells into the blood. Here we demonstrate a rapid acceleration of medullary thymocyte phenotypic maturation through loss of CD69 induced by sphingosine 1-phosphate (S1P) receptor agonist. Low nanomolar agonist concentrations selectively induce changes in CD69int CD62Lhigh single positive T cells, resulting in down-modulation of CD69 within 2 h. While CD69 loss is accelerated, egress of mature T cells into blood is inhibited >95% within 2 h. Both processes exhibit parallel sensitivities and dose–responses. Together, these data reveal a potent means for rapidly regulating thymic export where S1P receptor agonism alters both phenotypic maturation and egress of thymocytes into blood during late thymic maturation. The S1P system is now shown to acutely regulate both thymic and lymph node egress. Inhibition of lymphocyte egress from thymus and lymph node can contribute synergistically to clinically useful immunosupression by disrupting recirculation of peripheral T cells.
Sphingosine 1-phosphate (S1P) receptor agonists, such as the phosphate-ester metabolite of FTY720 (1, 2), are clinically useful immunosuppressants for transplantation rejection (3) and have recently been shown to regulate egress of naïve lymphocytes (4) and both CD4 (5) and CD8 (6) effector cells by retention of lymphocytes on the abluminal side of sinus-lining endothelium in lymph nodes but not spleen (4, 7). Long-term studies have also demonstrated effects on the emigration of thymocytes from thymus into blood and secondary lymphoid organs (8), suggesting that an important step in thymic egress is also affected.
FTY720 and its chiral analog 2-amino-4-(4-heptyloxyphenyl)-2-methylbutanol [AAL-(R)] are full agonists for four of five G protein-coupled receptors for S1P. Originally described as endothelial differentiation genes, or edg receptors, five cloned G protein-coupled high-affinity receptors (9) have been described for S1P that show low nanomolar binding affinities (10–14). They are closely related structurally to the receptors edg2/lpa1, edg4/lpa2, and edg7/lpa3 for lysophophatidic acid. They are expressed on endothelial cells (S1P1, S1P3), and mRNA can be detected in lymphocytes (S1P4, S1P5) (15, 16) and on vascular smooth muscle and myocardium (S1P2 and S1P1). Some investigators have suggested that S1P1 is also expressed in lymphocytes, although the presence of active receptor on uncultured lymphocytes remains controversial (17–19). Of these receptors, only S1P2 is not activated by the phosphoryl metabolite of FTY720 (1).
S1P1, S1P3, S1P5, and S1P4 receptors activate multiple cellular responses, through pertussis toxin (PTX)-sensitive i.e., Gi-coupled events, and PTX-independent transduction steps (activation of rac and rho small GTP-binding proteins) (20–23). The receptors play significant roles in cardiovascular physiology, regulating blood pressure (21) and heart rate (24, 25). S1P1 is essential for maturation of arterial media (26, 27). The receptors are found in caveolae and are associated with the regulation of other mediators such as NO that can regulate vascular caliber and pressor function (28, 29). It might therefore seem surprising that an acute regulator of vascular function should play a significant role in the control of lymphocyte egress.
The acuity of responses regulated by S1P receptors have led us to study short-term events occurring in thymic medulla. Whereas lymphocyte egress into lymph represents a process of egress across anatomically diffuse lymphoid sites, the thymus represents a single anatomical site where control of lymphocyte egress could have significant long-term impact on peripheral immune diversity, and thus immunosuppression (30, 31).
Thymic maturation and emigration are essential for the maintenance of peripheral T cell diversity. After positive selection and initiation of negative selection (32, 33) in the thymic cortex (34–36), single positive (SP) T cells cross the cortico-medullary junction by a PTX-inhibitable step that may include chemokines and their receptors, including CCR4 (37, 38), to spend ≈2 weeks in the medulla, where negative selection continues (39–42). Early medullary thymocytes express high levels of CD69 (43), while beginning to acquire CD62L (44, 45). Complete medullary maturation involves loss of CD69 among other phenotypic markers. CD69 is regarded functionally by some as an early activation marker (46–48), and evidence based on CD69 overexpression also points to CD69 as a thymic retention mechanism through its C-type lectin domain (47, 49). After attaining the late medullary T cell phenotype, T cells appear to undergo CCR7-dependent emigration across medullary endothelium (50) into blood (51, 52). The identification of the molecular gatekeepers and the kinetics with which they regulate thymic egress remain an important unresolved question in thymic biology. We have therefore studied the acute regulation of thymic maturation and emigration by using a potent and well-characterized S1P receptor agonist.
Materials and Methods
Compounds. AAL-(R) and (S) enantiomer of AAL-(R) [AAL-(S)] were the kind gift of Novartis Pharma (Volker Brinkmann). The synthesis and characterization are described in refs. 1 and 2, and additional biological properties are described in refs. 4 and 7. The compounds were administered in a volume of 0.1 ml by i.p. injection as a solution in 10% (vol/vol) DMSO in water. Vehicle controls received the equivalent volume of 10% DMSO in water. Liquid chromatography-MS (LC-MS) analysis of AAL-(R) and its phosphate-ester in mouse plasma was performed by Jonathan Chang and Tove Tuntland (Genomics Institute of the Novartis Foundation) as described (4). Free concentrations of the phosphate ester of AAL-(R) [AFD-(R)] in mouse plasma were measured by LC-MS after centrifugation of 20 nM AFD in mouse plasma at 600,000 × g. All plasma proteins were sedimented from the plasma water and concentrations in the protein-bound and free plasma water fractions assayed by LC-MS.
Flow Cytometry. Single-cell thymic suspensions were prepared by passage of tissues, from adult C57BL6 mice of 6–10 weeks of age, through a 40-μm sieve. Lymphocytes were further isolated by ammonium chloride lysis of red blood cells. Cells were subsequently washed in PBS/5% FBS, and all samples were adjusted to a cell count of 4 × 108 cells per ml before staining. Single-step staining with direct fluorphore conjugates was performed for all markers except CD69. The following antibody-fluorophore combinations were used for phenotypic analysis of thymocytes by flow cytometry: FITC-αCD62L (MEL14, provided by M.G.M.-W.), biotin-αCD69 (H1.2H3, M.G.M.-W.), Cy5PE-αCD8 (53-6.7, Pharmingen), Cy7PE-αB220 (RA3-6B2, Caltag, South San Francisco, CA), APL-αCD44 (Pgp-1; M.G.M.-W.), and Cy7-αCD4 (RM4-5, Caltag). Lymphocyte populations were measured by flow cytometry (FACS DiVa; Becton Dickinson) using cellquest software (Becton Dickinson), and then analyzed with flowjo (Treestar, San Carlos, CA). Gating on CD8+ SP T cells included TCR– immature cells, which were not separately analyzed.
Thymic Dose–Response and Lymphopenia Studies. Five-fold dose dilutions of AAL-(R) in a 10% DMSO/water (vol/vol) vehicle, ranging from 5 mg/kg to 0.008 mg/kg, were administered by i.p. injection in a volume of 0.1 ml. Thymi and EDTA anticoagulated mouse blood were collected at 4 h for quantitation of thymocyte phenotype and lymphopenia as described (4).
Intrathymic (IT) Labeling. Mice were anesthetized with Avertin (Aldrich Chemical), and thymocytes were labeled by IT injection of 10 μl of FITC (2 mg/ml) in normal saline per thymic lobe as described (8, 39). After IT injection and recovery from surgery for the times stated in the figures, mice were administered either the active S1P agonist prodrug AAL-(R) or its inactive stereoisomer AAL-(S) to measure the effects of compound on thymic emigration to spleen. The percentage of thymocytes labeled with FITC ranged from 50% to 95%. Mice that showed <50% labeling were discarded from the experiments. Thymic export was measured by quantitating the accumulation of CD4+ or CD8+ lymphocytes that were CD62Lbright and CD69– in spleen over 24 h. The comparison of both CD4 and CD8 recent thymic immigrants was performed to ensure that adequate data for both subsets could be derived from spleen. The yield of labeled recent thymic emigrants of both CD4 and CD8 subsets per 105 splenocytes was within the ranges reported by other investigators (8, 39). All animal studies were performed under veterinary supervision and approved by the Institutional Animal Care and Use Committee at Scripps.
Curve Fitting. Nonlinear regression dose–response curve fitting was performed by using prism 3.03 (GraphPad, San Diego). Comparison of groups within dose–response curves was performed by using ANOVA with the Tukey–Kramer multiple comparisons test. Comparisons between single groups was carried out by using the Student's t test where indicated.
Results
AFD-(R), the Phosphate Ester of the Chiral FTY720 Analog AAL-(R), Is the Sole Molecular Species Detectable in Mouse Plasma. Agonism of the S1P receptor system acutely alters lymphocyte trafficking, inhibiting the egress of lymphocytes from lymph node into lymph and causing almost maximal peripheral blood lymphopenia within 2–4 h (4). We therefore hypothesized that S1P agonist regulation of thymic egress occurred over the same time frame. To study thymic egress acutely, we assess the short-term effects of the (R)-isomer of AAL-(R) (1, 4, 7) on thymic function. AAL-(R), a chiral methyl analog of FTY720 (Fig. 1), is a potent immunosuppressive prodrug, producing lymphocyte sequestration in lymph nodes and peripheral blood lymphopenia once phosphorylated to its active form. Its phosphate-ester (AFD) is a low nanomolar full agonist on four of the G protein-coupled receptors for S1P (S1P1, S1P3, S1P4, and S1P5) but not S1P2 (4, 7), with a 1.26 nM EC50 for S1P1 in the GTPγS agonist assay.
Fig. 1.
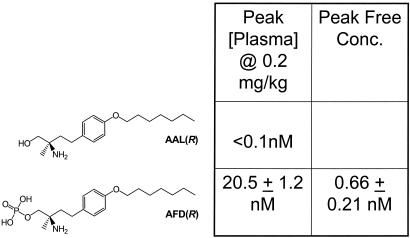
The structures of AAL-(R) and its phosphate-ester S1P receptor active agonist AFD-(R) are shown, together with total and free plasma concentrations in nmol/liter as determined by LC-MS as described in Materials and Methods.
AAL-(R) and AAL-(S) form a useful controlled pair of chemical reagents for in vivo manipulation of lymphocyte trafficking. After administration to mice AAL-(R) but not AAL-(S) is rapidly and quantitatively phosphorylated to AFD-(R) (Fig. 1). Assay of plasma concentrations of both AAL-(R) and AFD-(R) by LC-MS in mice dosed at 0.2 mg/kg (a dose inducing full lymphocyte sequestration), showed that levels of the parent amino-alcohol [AAL-(R)] were never above the limits of detection (0.1 nM), whereas the phosphorylated active metabolite AFD was present in serum at 11.7 ± 4.70 nM (mean ± SD) at 15 min, peaked at 1 h at 20.5 ± 1.23 nM, declining to 16.9 ± 3.80 nM at 4 h. AFD shares similar physical properties with the physiological ligand S1P, both with regard to receptor interactions and physical interactions with plasma proteins. Like S1P (53), AFD is significantly protein bound in mouse plasma with ≈3.2% free (Fig. 1). Thus AFD has its full range of biological effects on lymphocyte trafficking at subnanomolar concentrations of free ligand.
The S1P Receptor (R)-Isomer Agonist Rapidly Down-Modulates CD69 in a Defined Subset of SP Thymocytes. FTY720 administration has been reported to induce long-term changes over 5–20 days in thymus cellularity and phenotype, specifically accumulation of SP T cells and loss of double positive T cells, with increased cellularity within the medulla (8). However, we found that 4-h exposure to AAL-(R) produced no significant change in total numbers of thymocytes (data not shown) nor in the distribution of the major thymocyte subsets (CD4–8–, CD4+8+, CD4+8–, and CD8+4–; Fig. 2A).
Fig. 2.
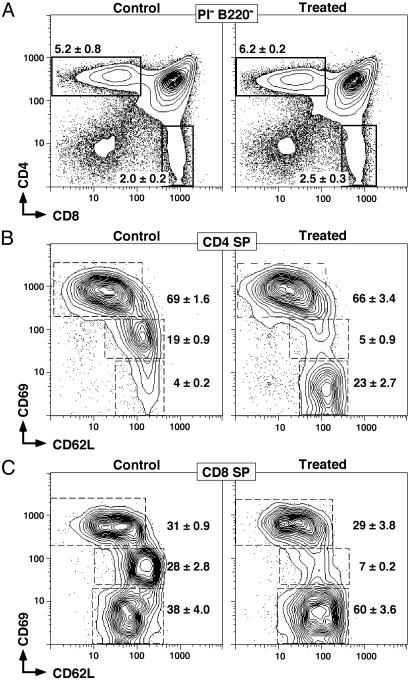
Six-color flow cytometric analysis of thymocytes. All profiles show cells negative for B220 and propidium iodide staining. (A) Gating for CD4 and CD SP populations is shown. The frequency (mean ± SD) of CD4+CD8+ double positive T cells was 85 ± 3.2%, and the frequency of CD4–CD8– was 3.3 ± 1.1% with no statistically significant differences seen over the first 24 h. The frequencies (means ± SD, n = 4) of the high, intermediate, and negative CD69 populations, together with CD62L expression levels, are shown for CD4 (B) and CD8 (C) SP T cells from mice treated with vehicle or 5 mg/kg AAL-(R) for 4 h before removal of thymi. Rapid AAL-(R)-dependent disappearance of the CD69int populations of both CD4 and CD8 SP cells is seen.
In contrast to the normal major thymocyte subset distributions, there were significant changes in the surface phenotype of both CD4 and CD8 SP thymocytes within 2–4 h (Fig. 2 B and C). Although there were no significant alterations in the number and frequency of early CD4+ medullary thymocytes (CD69high CD62Llow) (69 ± 1.6% control and 66 ± 3.4% treated, Student's t test, P = 0.34; Fig. 2B), there was almost complete loss of a CD69intermediate (CD69int) compartment (19 ± 0.9% in control) and concomitant gain of a medullary phenotype (CD69– CD62Lhigh)(4 ± 0.2% in control to 23 ± 2.7% after treatment, P = 0.006; Fig. 2B). A small residual population of CD69int cells (5 ± 0.9%) remained after treatment. The same trend was seen for CD8+ SP cells (Fig. 2C). Although more CD8 SP thymocytes resided in the late medullary compartment (CD69– CD62Lhigh) (38 ± 4.0% in control), the loss of the CD69int subset was clearly evident upon administration of the S1P receptor agonist (28 ± 2.8 control down to 7 ± 0.2 after treatment, P = 0.02 two-tailed t test). This phenotypic change was confined to the CD69int subset alone.
Total numbers of CD4 and CD8 SP thymocytes were not changed by exposure to AAL-(R), suggesting that phenotypic conversion occurs without loss of thymocytes or subsets of thymocytes. In addition, analysis of the number of CD69high SP cells for both CD4 and CD8 SP thymocytes (45) shows that this population remains constant and is not affected by administration of the S1P receptor agonist AAL-(R). Thus, the acute thymic effects of AAL-(R) administration appear to act distal to the CD69high SP population and induce loss of CD69 in the CD69int population.
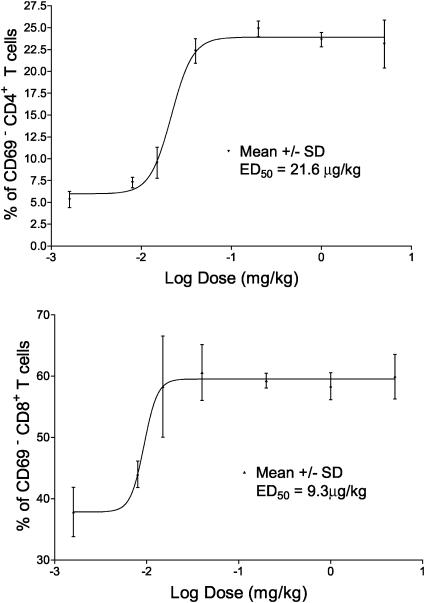
Selective and Potent S1P Receptor Agonist-Induced CD69 Down-Modulation. Maximal loss of CD69 from the CD69int populations of both CD4 and CD8 SP thymocytes was achieved after 2-h exposure to AAL-(R) (Fig. 2 A) with similar levels of conversion retained to at least 24 h (data not shown). Further, the inactive (S)-isomer of the agonist AAL had no effect and was comparable to results obtained with vehicle alone (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). The potency of conversion can be seen from the ED50 CD69int to CD69– conversion of CD4 SP and CD8 SP T cells at 4 h after AAL-(R) administration was 21.6 and 9.3 μg/kg, respectively (Fig. 3), whereas AAL-(S) was inactive at 5.0 mg/kg. Thus, the loss of CD69 expression in response to S1P receptor agonism is very rapid, selective, and highly sensitive.
Fig. 3.
The dose–responses of CD4 or CD8 SP CD62L+CD69– T cell frequencies were measured by flow cytometry after4hof AAL-(R) treatment, and the ED50 was calculated by nonlinear regression as described in Materials and Methods (mean ± SD; n = 4, R2 = 0.988).
The Rapidly Formed CD69– SP Populations Retain the Ly6C Phenotype of Thymocytes and Not Peripheral Lymphocytes. S1P agonists induce lymphocyte sequestration in secondary lymphoid organs (4), whereas activated T cells migrate from the periphery to the thymus in adult mice, and the circulating T cells migrate to the thymus in the neonate (54, 55). Thus it was possible that accumulation of CD69– CD62Lhigh SP thymocytes was simply the result of thymic sequestration of T cells from the periphery. We used the differential expression of the surface glycoprotein Ly6C across SP thymocytes and peripheral T cells to resolve this issue directly (Fig. 7, which is published as supporting information on the PNAS web site) by flow cytometric analysis of both CD69int and CD69– SP T cells. The distribution of Ly6C on SP CD4 thymocytes in treated animals (15%) is statistically equivalent to the distribution in untreated mice (14%). Similar findings were found upon analysis of CD8 SP thymocytes, of which 4% were Ly6C positive in treated mice compared with 5% in controls. Ly6C expression in thymocytes was substantially different to its distribution in peripheral T cells, of which 43% CD4 SP and 46% CD8 SP were positive. Therefore, it is most likely that the accumulation of CD69– cells in the thymus was caused by phenotype, rather than the trapping of peripheral T cells.
AAL-(R) Induced Rapid Inhibition of Thymic Emigration. To evaluate the effects of an S1P receptor agonist on thymic egress, we used direct FITC conjugation of thymocytes by IT injection as described (8, 39). Mice received either AAL-(R) or AAL-(S) by i.p. injection 1 h after IT labeling. Fifty percent to >95% of CD4 and CD8 SP thymocytes were FITC-labeled (Fig. 4 A and B, respectively). A total of 1,905 ± 400 CD4 SP (Fig. 4A) and 1,051 ± 176 CD8 SP (Fig. 4B) recent thymic emigrants were recruited to spleen per 5 × 105 splenocytes, respectively over the first 24 h in both vehicle control or AAL-(S)-treated mice (5 mg/kg).
Fig. 4.
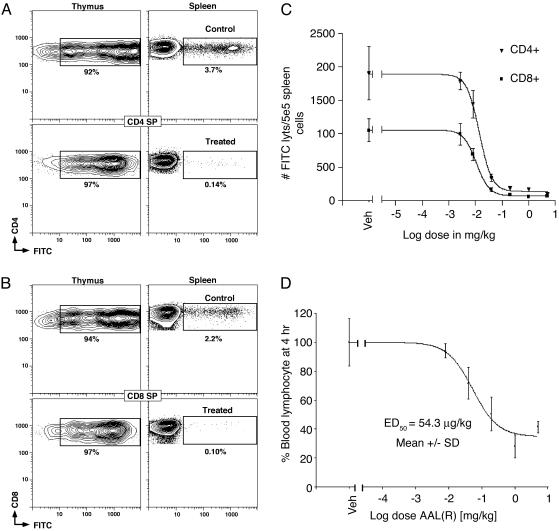
Inhibition of thymic egress by S1P receptor agonist. AAL-(R) or vehicle was administered 1 h after IT injection of FITC. The migration of recent thymic CD4 SP (A) and CD8 SP (B) emigrants to spleen was measured by flow cytometry 24 h after IT labeling (n = 5, mean ± SD). FITC was used to monitor thymic emigrants, and APC-αCD62L (MEL14) replaced APC-αCD44. AAL-(S) at 5 mg/kg was not statistically different to vehicle control. The dose–responses for inhibition of thymic egress of both CD4 and CD8 SP T cells by AAL-(R) (R2 = 0.996) (C) were similar in potency and steepness to the loss of CD69 (Fig. 3B). Mice had similar numbers of total T cells. (D) Dose–response for peripheral blood lymphopenia induced by AAL-(R).
Doses of AAL-(R) as low as 0.04 mg/kg inhibited thymic emigration to spleen by 82.4% and 84.5% for CD4 SP (ED50 = 14 μg/kg) and CD8 SP cells (ED50 = 10 μg/kg), respectively (Fig. 4C). Inhibition occurred rapidly (>95% inhibition with compound dosed 1 h after recovery from IT labeling). The dose–responses for inhibition of thymic emigration (Fig. 4C) were more sensitive than the induction of blood lymphopenia (Fig. 4D; ED50 = 54.8 μg/kg). In fact, thymic egress has been >80% inhibited at doses of AAL-(R) (0.04 mg/kg) that sustain blood lymphocyte levels that are not significantly changed from vehicle (P = 0.112). Thus thymic egress is potently, selectively, and almost completely inhibited by AAL-(R) and not AAL-(S), when assayed by this method.
Discussion
Evidence is presented for potent, rapid, and selective acceleration of medullary thymocyte phenotypic maturation as measured by loss of CD69 from the CD69int population of SP thymocytes. In addition, there is potent and selective inhibition of thymic emigration by administration of the potent S1P receptor agonist AAL-(R).
These data place two discrete steps regulated by S1P receptor agonists sequentially within a model of medullary function (Fig. 5). AAL-(R) induces CD69 loss from CD69int but not CD69high SP T cells that are already CD62L+, defining a maturational step in medulla occurring independently of accumulation of CD62L+ cells over time. S1P receptor activation thus does not act on T cells before crossing of the cortico-medullary junction. In medulla, we functionally define a population of CD69int SP cells, distal to CD69high SP T cells, that is uniquely susceptible either directly or indirectly to S1P receptor agonist-regulated loss of CD69. In contrast to studies (8) at 20 days, in which complex homeostatic regulation has occurred, numbers of thymic CD69high SP cells are unaffected by AAL-(R), as are total SP cell numbers. These data together with the inhibition of the known CCR7-dependent final thymic egress (51) on IT labeling are compatible only with a sequential model in which acceleration of CD69 loss precedes inhibition of thymic egress into blood. S1P receptor agonists thus have complex and apparently paradoxical effects in thymic medulla. Within 2 h, agonist expands a reservoir of phenotypically mature naïve cells, while restraining their release. A number of potential explanations are worthy of consideration. The CD69int population may be a rapid biological sensor to endogenous ligand S1P generated early in innate inflammatory processes. Sphingosine kinase is activated by both IL-1β and tumor necrosis factor α stimulation, resulting in local S1P production and secretion (56). Thymus can therefore respond in minutes to hours to peripheral inflammation by mobilizing this indicator population.
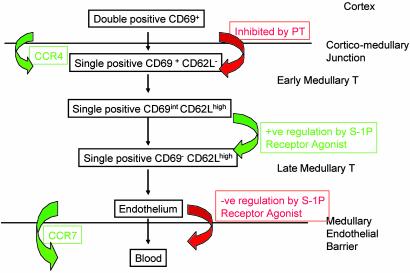
Fig. 5.
Shown are the sequence of S1P receptor-regulated events in thymic maturation and egress based on phenotypic (43, 66) and positional changes within medullary populations (37, 51), the effects of PTX (38, 52), and formal evidence for the inhibition of thymic emigration. Double positive CD69+ T cells are found in the cortex, whereas SP CD69+ T cells are found in the medulla except in the presence of PTX when SP CD69+ T cells remain in the cortex. An S1P receptor activation-independent step results in generation of a population of CD69int SP T cells, which then undergo rapid maturation to CD69– SP cells induced by S1P receptor agonism. The egress of all mature cells is then rapidly negatively regulated by S1P receptor activation, inhibiting the CCR7-dependent appearance of mature cells in secondary lymphoid organs.
Acceleration of CD69 loss, a potential thymic retention mechanism (47, 49), may promote T cell egress once acute inflammatory events have subsided. This could occur even without complete medullary maturation and negative selection. Additional numbers of naïve cells would help support an adaptive response. The two steps influenced by S1P agonism reveal an upstream point of control. Stability of CD69high SP cell numbers suggests that conversion from CD69high to CD69int SP may require extrinsic regulation.
Key findings of these studies were not anticipated by previous studies (8). These impact on thymic regulation and the broad understanding of S1P, its mimics, and receptors in tissue homeostasis. These include: (i) functional definition of a population of rapidly S1P agonist responsive medullary T cells by high-resolution flow cytometry that represents an indicator population within medulla, (ii) the rapidity of S1P receptor agonist-induced phenotypic change in medullary thymocytes (2 h versus 20 days), (iii) the coordinate dose–response and kinetics for egress inhibition and phenotypic alteration, and (iv) formal demonstration that alterations in thymic function occur at low nanomolar total plasma concentration (picomolar free concentrations) of agonist, which together with the steep dose–response curves, shed light on a threshold mechanism of lipid agonist regulation in thymus. Peak free agonist concentrations are below the EC50 for receptor activation. Thymic regulation occurs at low receptor occupancy, and this provides a medically important physiological mechanism for differentiating between useful immune regulation and the potentially deleterious pressor and cardiac functions of S1P mimics (56).
Loss of CD69 in the CD69int SP T cells does not reflect an apoptotic response to an FTY720 analog. First, the constant numbers of viable SP thymocytes during the course of these experiments, even over 24 h, point to the phenotypic alteration of CD69 as being phenotypic maturation within the preexisting SP population and rule out the possibility of the induction of apoptotic effects in medullary thymocyte subsets. Second, FTY720 only induces apoptosis at in vitro concentration of 10 μM (57–61), concentrations that are 1,000-fold higher than those needed for the induction of thymic phenotypic change or the inhibition of thymic emigration.
Low doses of AAL-(R) (0.04–0.2 mg/kg) induce inhibition of thymic egress and enhanced CD69 loss and lymphopenia at total plasma concentrations of AFD in the low nanomolar range and free concentrations in the pM range (Fig. 1). Thymic effects and lymphopenia in vivo seem to have different dose–responses than the direct S1P and FTY720 effects on cultured and uncultured lymphocytes or lymphocytic cell lines (17–19). Neither direct effects on lymphocytes nor indirect regulation of lymphocytes secondary to alterations in stromal or endothelial barriers have been resolved experimentally. The distribution of S1P receptors upon lymphocytes and stromal cells and their contribution(s) directly or indirectly to regulation of CD69 loss and thymic or lymph node egress are an important mechanistic challenge to the field.
Significant amplification of the AAL-(R) agonist signal is suggested by the steepness of the dose–response curves for both CD69 loss and inhibition of thymic emigration, which do not show a standard relationship (i.e., a 10–90% change over an 81-fold ligand concentration range). Lymphopenia in vivo correlates with receptor activation in vitro for the nonhydrolyzable S1P receptor agonist FTY-phosphonate (4), and receptor-coupled amplification is expected. A threshold of S1P receptor activation thus acts as a biological on switch at low nanomolar concentrations of agonist. This is seen in many agonist-driven processes such as threshold T cell receptor occupancy needed to trigger clonal expansion (62) or activation of responses to serotonin or ryanodine (63–65).
The rapid inhibition of egress from lymph node to lymphatic (4), as well as from thymus to blood, therefore share a similar molecular gatekeeper, in that both processes are inhibited by potent ligands activating the S1P receptors. The speed and completeness of this inhibition suggest that a shared process such as endothelial barrier regulation both in thymus and lymph node could be a critical regulator of T cell trafficking. Inhibiting egress of naive and effector T cells from lymph nodes and mature thymocytes from thymus when achieved over time by long-term compound administration could contribute synergistically to therapeutically useful immunosuppression.
Supplementary Material
Acknowledgments
H.R. thanks Richard Lerner and Jeff Kelly for support and counsel and Jonathan Sprent for his critical review of the manuscript. H.R. is supported by The Scripps Research Institute. C.D.S. was supported by U.S. Public Health Service Grants AI41079, AI45809, and AG20186 and is a Scholar of the Leukemia and Lymphoma Society. M.G.M.-W. was supported by U.S. Public Health Service Grants AI40215 and AI47231.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: S1P, sphingosine 1-phosphate; SP, single positive; LC-MS, liquid chromatography-MS; AAL-(R), 2-amino-4-(4-heptyloxyphenyl)-2-methylbutanol; AAL-(S), (S) enantiomer of AAL-(R); AFD, phosphate ester of AAL; IT, intrathymic; PTX, pertussis toxin.
References
- 1.Kiuchi, M., Adachi, K., Kohara, T., Minoguchi, M., Hanano, T., Aoki, Y., Mishina, T., Arita, M., Nakao, N., Ohtsuki, N., et al. (2000) J. Med. Chem. 43, 2946–2961. [DOI] [PubMed] [Google Scholar]
- 2.Kiuchi, M., Adachi, K., Kohara, T., Teshima, K., Masubuchi, Y., Mishina, T. & Fujita, T. (1998) Bioorg. Med. Chem. Lett. 8, 101–106. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann, V. & Lynch, K. (2002) Curr. Opin. Immunol. 14, 569–575. [DOI] [PubMed] [Google Scholar]
- 4.Mandala, S., Hajdu, R., Bergstrom, J., Quackenbush, E., Xie, J., Milligan, J., Thornton, R., Shei, G.-J., Card, D., Keohane, C., et al. (2002) Science 296, 346–349. [DOI] [PubMed] [Google Scholar]
- 5.Xie, J., Nomura, N., Quackenbush, E., Forrest, M. & Rosen, H. (2003) J. Immunol. 170, 3662–3670. [DOI] [PubMed] [Google Scholar]
- 6.Pinschewer, D. D., Ochsenbein, A. F., Odermatt, B., Brinkmann, V., Hengartner, H. & Zinkernagel, R. M. (2000) J. Immunol. 164, 5761–5770. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann, V., Davis, M. D., Heise, C. E., Albert, R., Cottens, S., Hof, R., Bruns, C., Prieschl, E., Baumruker, T., Hiestand, P., et al. (2002) J. Biol. Chem. 277, 21453–21457. [DOI] [PubMed] [Google Scholar]
- 8.Yagi, H., Kamba, R., Chiba, K., Soga, H., Yaguchi, K., Nakamura, M. & Itoh, T. (2000) Eur. J. Immunol. 30, 1435–1444. [DOI] [PubMed] [Google Scholar]
- 9.Chun, J., Goetzl, E., Hla, T., Ingarashi, Y., Lynch, K., Moolenaar, W., Pyne, S. & Tigyi, G. (2002) Pharmacol. Rev. 54, 265–269. [DOI] [PubMed] [Google Scholar]
- 10.Lynch, K. R. (2002) Biochim. Biophys. Acta 1582, 70–71. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima, N., Ishii, I., Contos, J. J. A., Weiner, J. A. & Chun, J. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 507–534. [DOI] [PubMed] [Google Scholar]
- 12.Hla, T., Lee, M.-J., Ancellin, N., Paik, J. H. & Kluk, M. J. (2001) Science 294, 1875–1878. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel, S. (2000) Ann. N.Y. Acad. Sci. 905, 54–60. [DOI] [PubMed] [Google Scholar]
- 14.Van Brocklyn, J. R. & Spiegel, S. (2000) Methods Enzymol. 312, 401–406. [DOI] [PubMed] [Google Scholar]
- 15.Graeler, M. H., Bernhardt, G. & Lipp, M. (1999) Curr. Top. Microbiol. Immunol. 246, 131–136. [DOI] [PubMed] [Google Scholar]
- 16.Im, D. S., Heise, C. E., Ancellin, N., O'Dowd, B. F., Shei, G. J., Heavens, R. P., Rigby, M. R., Hla, T., Mandala, S., McAllister, G., et al. (2000) J. Biol. Chem. 275, 14281–14286. [DOI] [PubMed] [Google Scholar]
- 17.Graeler, M. & Goetzl, E. (2002) FASEB 16, 1874–1878. [DOI] [PubMed] [Google Scholar]
- 18.Graeler, M., Shankar, G. & Goetzl, E. (2002) J. Immunol. 169, 4084–4087. [DOI] [PubMed] [Google Scholar]
- 19.Graler, M. & Goetzl, E. (2002) Biochim. Biophys. Acta 1582, 168–174. [DOI] [PubMed] [Google Scholar]
- 20.Ancellin, N. & Hla, T. (2000) Ann. N.Y. Acad. Sci. 905, 260–262. [DOI] [PubMed] [Google Scholar]
- 21.Pyne, S. & Pyne, N. J. (2000) Biochem. J. 349, 385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moolenaar, W. H. (1999) Exp. Cell Res. 253, 230–238. [DOI] [PubMed] [Google Scholar]
- 23.Wells, C. D., Gutowski, S., Bollag, G. & Sternweis, P. C. (2001) J. Biol. Chem. 276, 28897–28905. [DOI] [PubMed] [Google Scholar]
- 24.Liliom, K., Sun, G., Bunemann, M., Virag, T., Nusser, N., Baker, D. L., Wang, D., Fabian, M. J., Brandts, B., Bender, K., et al. (2001) Biochem. J. 355, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama, A., Yatomi, Y., Ozaki, Y. & Hashimoto, K. (2000) Cardiovasc. Res. 46, 119–125. [DOI] [PubMed] [Google Scholar]
- 26.Liu, Y., Wada, R., Yamashita, T., Mi, Y., Deng, C. X., Hobson, J. P., Rosenfeldt, H. M., Nava, V. E., Chae, S. S., Lee, M. J., et al. (2000) J. Clin. Invest. 106, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allende, M. L. & Proia, R. L. (2002) Biochim. Biophys. Acta 1582, 222–227. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi, J. & Michel, T. (2000) J. Biol. Chem. 275, 32363–32370. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi, J. & Michel, T. (2001) J. Biol. Chem. 276, 36281–36288. [DOI] [PubMed] [Google Scholar]
- 30.Dumont, F. J. (1996) Princ. Drug Dev. Transplant. Autoimmunity 1, 175–205. [Google Scholar]
- 31.Waldmann, H. & Cobbold, S. (1998) Annu. Rev. Immunol. 16, 619–644. [DOI] [PubMed] [Google Scholar]
- 32.Ernst, B., Surh, C. & Sprent, J. (1995) J. Exp. Med. 182, 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surh, C. & Sprent, J. (1994) Nature 372, 100–103. [DOI] [PubMed] [Google Scholar]
- 34.Lind, E. F., Prockop, S. E., Porritt, H. E. & Petrie, H. T. (2001) J. Exp. Med. 194, 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berzins, S. P., Boyd, R. L. & Miller, J. F. A. P. (1998) J. Exp. Med. 187, 1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berzins, S. P., Godfrey, D. I. & Miller, J. F. A. P. (1999) Proc. Natl. Acad. Sci. USA 96, 9787–9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poznansky, M. C., Olszak, I. T., Evans, R. H., Wang, Z., Foxall, R. B., Olson, D. P., Weibrecht, K., Luster, A. D. & Scadden, D. T. (2002) J. Clin. Invest. 109, 1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki, G., Sawa, H., Kobayashi, Y., Nakata, Y., Nakagawa, K.-i., Uzawa, A., Sakiyama, H., Kakinuma, S., Iwabuchi, K. & Nagashima, K. (1999) J. Immunol. 162, 5981–5985. [PubMed] [Google Scholar]
- 39.Scollay, R. G., Butcher, E. C. & Weissman, I. L. (1980) Eur. J. Immunol. 10, 210–218. [DOI] [PubMed] [Google Scholar]
- 40.Tough, D. F. & Sprent, J. (1995) Immunol. Today 16, 273–274. [Google Scholar]
- 41.Tough, D. F. & Sprent, J. (1994) J. Exp. Med. 179, 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scollay, R. & Godfrey, D. I. (1995) Immunol. Today 16, 268–273. [DOI] [PubMed] [Google Scholar]
- 43.Campbell, J. J., Pan, J. & Butcher, E. C. (1999) J. Immunol. 163, 2353–2357. [PubMed] [Google Scholar]
- 44.Ge, Q. & Chen, W. F. (1999) Immunology 97, 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian, T., Zhang, J., Gao, L., Qian, P. Q. & Chen, W.-F. (2000) Int. Immunol. 13, 313–320. [DOI] [PubMed] [Google Scholar]
- 46.Hare, K. J., Jenkinson, E. J. & Anderson, G. (1999) J. Immunol. 162, 3978–3983. [PubMed] [Google Scholar]
- 47.Nakayama, T., Kasprowicz, D. J., Yamashita, M., Schubert, L. A., Gillard, G., Kimura, M., Didierlaurent, A., Koseki, H. & Ziegler, S. F. (2002) J. Immunol. 168, 87–94. [DOI] [PubMed] [Google Scholar]
- 48.Lauzurica, P., Sancho, D., Torres, M., Albella, B., Marazuela, M., Merino, T., Bueren, J. A., Martinez-A, C. & Sanchez-Madrid, F. (2000) Immunobiology 95, 2312–2320. [PubMed] [Google Scholar]
- 49.Feng, C., Woodside, K. J., Vance, B. A., El-Khoury, D., Canelles, M., Lee, J., Gress, R., Fowlkes, B. J., Shores, E. W. & Love, P. E. (2002) Int. Immunol. 14, 535–544. [DOI] [PubMed] [Google Scholar]
- 50.Anderson, M., Anderson, S. K. & Farr, A. G. (2000) Int. Immunol. 12, 1105–1110. [DOI] [PubMed] [Google Scholar]
- 51.Ueno, T., Hara, K., Willis, M. S., Malin, M. A., Hopken, U. E., Gray, D. H. D., Matsushima, K., Lipp, M., Springer, T. A., Boyd, R. L., et al. (2002) Immunity 16, 205–218. [DOI] [PubMed] [Google Scholar]
- 52.Chaffin, K. E. & Perimutter, R. M. (1991) Eur. J. Immunol. 21, 2565–2573. [DOI] [PubMed] [Google Scholar]
- 53.Murata, N., Sato, K., Tomura, H., Kon, J., Yanagita, M., Kuwabara, A., Ul, M. & Okajima, F. (2000) Biochem. J. 352, 809–815. [PMC free article] [PubMed] [Google Scholar]
- 54.Agus, D., Surh, C. & Sprent, J. (1991) J. Exp. Med. 173, 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Surh, C., Sprent, J. & Webb, S. (1993) J. Exp. Med. 177, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spiegel, S. & Milstien, M. (2003) Nat. Rev. Mol. Cell Biol. 4, 397–407. [DOI] [PubMed] [Google Scholar]
- 57.Nagahara, Y., Ikekita, M. & Shinomiya, T. (2000) J. Immunol. 165, 3250–3259. [DOI] [PubMed] [Google Scholar]
- 58.Li, X. K., Shinomiya, T., Enosawa, S., Kakefuda, T., Amemiya, H. & Suzuki, S. (1997) Transplant. Proc. 29, 1267–1268. [DOI] [PubMed] [Google Scholar]
- 59.Fujino, M., Li, X., Guo, L., Kitazawa, Y., Funeshima, N., Fukada, S., Kimura, H., Miyashita, T., Okuyama, T., Amano, T. & Suzuki, S. (2001) Transplant. Proc. 33, 3084–3085. [DOI] [PubMed] [Google Scholar]
- 60.Fujino, M., Li, X., Guo, L., Amano, T. & Suzuki, S. (2001) Int. Immunopharmacol. 1, 2011–2021. [DOI] [PubMed] [Google Scholar]
- 61.Fujino, M., Li, X., Kitazawa, Y., Guo, L., Kawasaki, M., Funeshima, N., Amano, T. & Suzuki, S. (2002) J. Pharmacol. Exp. Ther. 300, 939–945. [DOI] [PubMed] [Google Scholar]
- 62.Williams, C. B., Engle, D. L., Kersh, G. J., White, J. M. & Allen, P. M. (1999) J. Exp. Med. 189, 1531–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hohenegger, M., Berg, I., Weigl, L., Mayr, G., Potter, B. & Guse, A. (1999) Br. J. Pharmacol. 128, 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Testorf, M., Karlsson, A., Svensson, S., Oberg, P. & Lundstrom, I. (2001) Biophys. Chem. 94, 1–9. [DOI] [PubMed] [Google Scholar]
- 65.Kilbourne, E. & Winneker, R. (2001) Thromb. Haemostasis 85, 924–928. [PubMed] [Google Scholar]
- 66.Cyster, J. G. (2002) J. Clin. Invest. 109, 1011–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.