Abstract
SPA-1 is a principal Rap1 GTPase-activating protein in the hematopoietic progenitors and peripheral T cells, and SPA-1-deficient mice develop a spectrum of myeloproliferative stem cell disorders of late onset. In the present study, we show that SPA-1-deficient mice develop age-dependent T cell unresponsiveness preceding the myeloid disorders, whereas the T cell numbers remained unchanged. Progression of the T cell dysfunction was attributed to the age-dependent increase in CD44high T cell population that was unresponsive to T cell receptor stimulation. Younger SPA-1-deficient mice exhibited selectively impaired recall T cell responses against a T-dependent antigen with normal primary antibody response. These results suggested that the unresponsiveness of CD44high T cells was antigen-driven in vivo. T cells from younger SPA-1–/– mice showed much greater and more persisted Rap1 activation by anti-CD3 stimulation than control T cells. Furthermore, freshly isolated T cells from SPA-1–/– mice exhibited progressive accumulation of Rap1GTP as mice aged. T cells from aged SPA-1–/– mice with high amounts of Rap1GTP showed normal or even enhanced Ras activation with little extracellular signal-regulated kinase activation in response to anti-CD3 stimulation, indicating that excess Rap1GTP induced the uncoupling of Ras-mediated extracellular signal-regulated kinase activation. These results suggested that antigenic activation of naïve T cells in SPA-1–/– mice was followed by anergic rather than memory state due to the defective down-regulation of Rap1 activation, resulting in the age-dependent progression of overall T cell immunodeficiency.
Full activation of T cells by antigens leading to the proliferation and cytokine production depends on the orchestrated interaction with specific antigen-presenting cells called immunological synapse, which involves a number of costimulatory and cell adhesion molecules (1, 2). Default in cell-adhesive or costimulatory signaling during the process may result in either inefficient T cell activation or irreversible T cell unresponsiveness called anergy (3, 4). Among a number of the critical molecules, β2-integrin LFA-1 plays an important role in the initiation of immunological synapse formation (5, 6). T cell receptor (TCR)-mediated recognition of a very small number of MHC/peptide complexes on antigen-presenting cells triggers the LFA-1–ICAM-1 interaction via inside-out activation, leading to the formation of macromolecular structures for efficient cell signaling (5).
Rap1 is a potent activator of integrins (7–9), and evidence has been accumulated that Rap1 signaling plays an important role in regulating various integrin-mediated cellular functions (reviewed in ref. 10). In T cells, stimulation of antigen receptors induced rapid activation of Rap1 (11), which was essential for the immunological synapse formation with antigen-presenting cells presenting the specific antigens (12). On the other hand, it was reported that anergic T cell clones generated in vitro by antigen stimulation unaccompanied by a costimulatory signal contained unusually high amounts of Rap1GTP (13) and that overexpression of a dominant active mutant of Rap1, RapV12, induced T cell anergy in vitro (12, 13). More recently, on the other hand, it was reported that RapV12 transgenic mice showed no evidence of anergy with the T cell responses being rather enhanced via integrin activation (14). Thus, although it is likely that Rap1 signaling plays a significant role in the T cell activation, its involvement in the T cell unresponsiveness or anergy remains controversial.
Rap1 signaling is controlled by a balance between guanine nucleotide exchange factors catalyzing the Rap1 activation through coupling with various receptors and GTPase-activating proteins (GAPs) enhancing the inactivation of Rap1 (10, 15). SPA-1 is a predominant Rap1GAP expressed in the lymphohematopoietic tissues (16, 17). Recently, we generated SPA-1 gene-deficient (SPA-1–/–) mice and found that they developed a spectrum of myeloid disorders that resembled human chronic myelogenous leukemia in the chronic phase, chronic myelogenous leukemia in blast crisis, or myelodysplastic syndrome (18). In the present study, we show that preceding the development of myeloid disorders, SPA-1–/– mice develop an age-dependent progression of T cell unresponsiveness due to the antigen-driven increase in anergic T cells with large amounts of Rap1GTP.
Materials and Methods
SPA-1-Deficient Mice. E14 embryonic stem cells were transfected with a linearized SPA-1 gene-targeting vector, in which a region covering exons 5–8 was replaced by a 1.8-kb fragment containing a pgk-neo cassette, microinjected into C57BL/6 (B6) blastocysts, and the resulting chimeras were mated with B6 mice (18). Heterozygous offspring were intercrossed to produce homozygous mutant mice, and those with mixed genetic backgrounds were used.
Cells and Cultures. Lymphocytes were cultured at 106 cells per well in the RPMI medium 1640 supplemented with 10% FCS in the presence of Con A (1–4 μg/ml), phorbol 12-myristate 13-acetate (PMA, 10 ng/ml) plus ionomycin (5 μg/ml), keyhole limpet hemocyanin (KLH), or 2,4,6-trinitrophenyl (TNP-KLH, 0.01–1 mg/ml) for 2 or 4 days, respectively, and pulsed with [3H]thymidine. For antigen receptor stimulation in vitro, T cells were incubated with anti-CD3 antibody (5 μg/ml) followed by anti-hamster IgG (5 μg/ml) and B cells with anti-IgM antibody (20 μg/ml). To separate T and B cells, spleen cell suspensions were incubated with phycoerythrin-conjugated anti-CD3 or anti-B220 antibody followed by anti-phycoerythrin-conjugated magnetic beads and separated using AutoMax bead columns (Miltenyi Biotec, Bergisch Gladbach, Germany). CD44high and CD44low T cell populations were obtained by sorting with a cell sorter (FACSVantage, Becton Dickinson). IL-2 activity was assessed by CTLL-2 cell assay.
Flow-Cytometric Analysis. Multicolor flow-cytometric analysis was performed by using a FACScan (Becton Dickinson) as described (19). Antibodies included anti-CD3, anti-CD4, anti-CD5, anti-CD8, anti-CD25, anti-CD69, and anti-CD44 (Pharmingen).
Thymic Organ Culture. Sorted lin– c-kit high Sca-1+ BM cells were cultured with deoxyguanosine-treated B6 fetal thymic lobes (100 cells per lobe) for 3 weeks under high oxygen submersion conditions as described (19).
Immunoblotting and Pull-Down Assay. Cells were lysed with lysis buffer (0.5% Triton X-100/150 mM NaCl/50 mM Tris·HCl, pH 7.6, and protease and phosphatase inhibitors) and blotted with anti-SPA-1 (7), anti-rapGAP, anti-phosphorylated extracellular signal-regulated kinase (ERK), or anti-Rap1 (Santa Cruz Biotechnology) as before (7). To detect Rap1GTP and RasGTP, cell lysates (0.5 to 1-mg proteins) were precipitated with GST-RalGDS-RBD and GST-c-Raf-1-RBD coupled with Sepharose beads followed by immunoblotting with anti-Rap1 and anti-Ras antibodies, respectively (7).
Antibody Response. Anti-KLH and anti-2,4-dinitrophenyl (DNP) antibodies in the sera and culture supernatants were assayed with ELISA.
Statistic Analysis. Statistic analysis was done by Student's t test.
Results
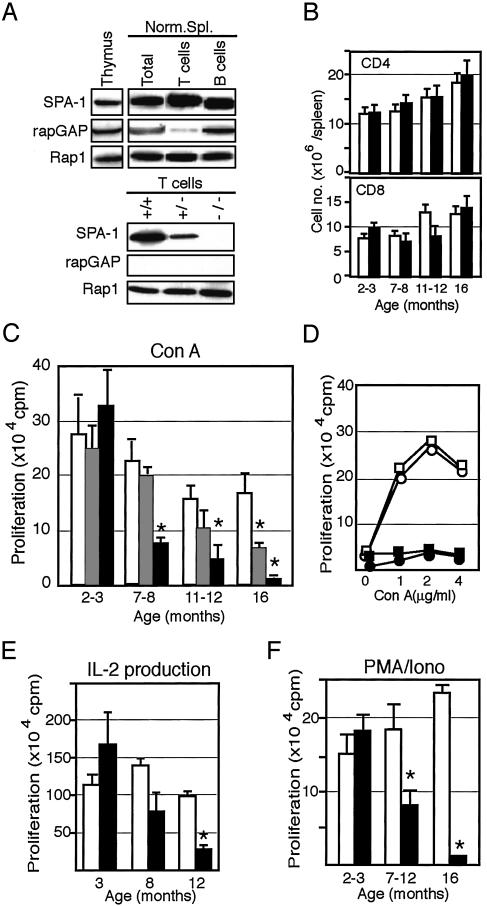
Age-Dependent Progression of T Cell Unresponsiveness in SPA-1-Deficient Mice. Both SPA-1 and rapGAP, two representative types of Rap1 GAPs, were expressed in the thymus and spleen cells. Splenic T cells, however, preferentially expressed SPA-1 with negligible rapGAP, whereas thymocytes and splenic B cells contained both SPA-1 and rapGAP, indicating that SPA-1 was a principal GAP for Rap1 in the peripheral T cells (Fig. 1A). T cells from SPA-1–/– mice showed no expression of SPA-1 of 130 kDa, and those from SPA-1+/– mice did roughly half a level of the control (Fig. 1 A). Ninety-five percent of SPA-1–/– mice developed myeloid disorders by 16 months, with the initial signs becoming evident after 12 months (18). In the present cohort, SPA-1–/– mice of various ages that showed no sign of the myeloid diseases by blood analysis and autopsies until 12 months and rare three 16-month-old mice without the diseases were used. Both absolute numbers and relative proportions of CD4 and CD8 T cells were comparable to those in the control littermates until 16 months (Fig. 1B). However, proliferative response to Con A in SPA-1–/– mice was reduced progressively from around half a year onward, whereas that in 2- to 3-month-old SPA-1–/– mice was comparable to or even higher than that in the control mice (Fig. 1C). In SPA-1+/– T cells, significant reduction in Con A response was evident only as late as 16 months (Fig. 1C). The reduced Con A response was not due to the shift of dose responses nor was restored by the addition of IL-2 (Fig. 1D). IL-2 production in response to Con A was also reduced in an age-dependent manner, whereas T cells from 3-month-old SPA-1–/– mice again tended to produce more IL-2 than the control (Fig. 1E). Furthermore, T cells from the aged SPA-1–/– T cells showed significantly reduced proliferation in response to PMA plus ionomycin as well, indicating that the effect was T cell autonomous (Fig. 1F). On the other hand, the B cell response to LPS remained largely unchanged at least until 12 months (213 ± 30 kcpm at 4 months and 114 ± 6.8 kcpm at 12 months in SPA-1–/– mice vs. 216 ± 29 kcpm at 4 months and 141 ± 5.5 kcpm at 12 months in SPA-1+/+ mice).
Fig. 1.
Age-dependent decrease of T cell responsiveness in SPA-1–/– mice. (A) Various lymphoid cells from normal B6 mice as well as T cells from SPA-1+/+, SPA-1+/–, and SPA-1–/– littermates were immunoblotted with the indicated antibodies. (B and C) CD4+ and CD8+ cell numbers (B) and maximal Con A response (C) of the spleen cells from SPA-1+/+ (open columns), SPA-1+/– (hatched columns), and SPA-1–/– (filled columns) mice of varying ages. Data are the means and SE of four (2–3 months), eight (7–8 months), eight (11–12 months), and three (16 months) mice. *, P < 0.05. (D) Proliferation of the spleen cells from 10-month-old SPA-1+/+ (○ and □) and SPA-1–/– (• and ▪) mice cultured with varying concentrations of Con A in the absence (○ and •) or presence (□ and ▪) of 100 units/ml IL-2. The means of triplicate cultures are shown. (E) IL-2 production of the spleen cells from SPA-1+/+ (open columns) and SPA-1–/– (filled columns) mice of varying ages in response to Con A. Data are the means and SE of three mice per each group. *, P < 0.05. (F) Proliferation of the spleen cells from SPA-1+/+ (open columns) and SPA-1–/– (filled columns) mice of varying ages in response to PMA plus ionomycin. Data are the means and SE of four (2–3 months), eight (7–12 months), and three (16 months) mice. *, P < 0.05.
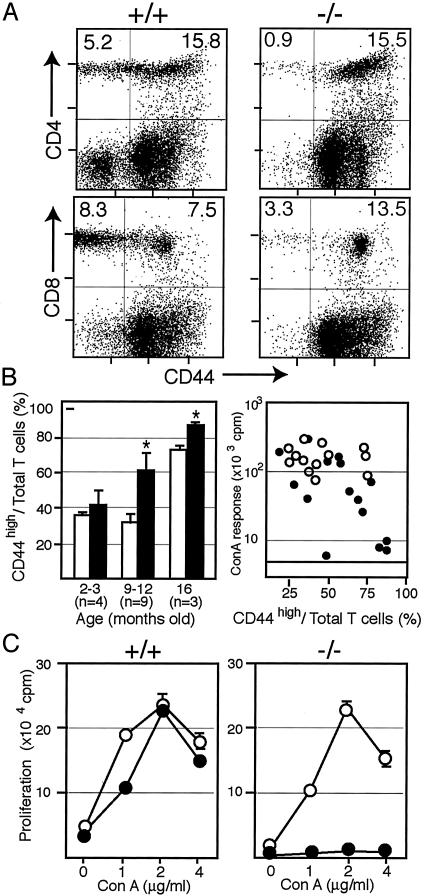
Age-Dependent Increase in CD44high T Cell Population with Impaired TCR Responsiveness in SPA-1–/– Mice. Because the results suggested the age-dependent increase in TCR-unresponsive T cells in SPA-1–/– mice, we attempted to characterize them with cell markers. Among a number of T cell markers, it was revealed that CD44high T cells were increased in the aged SPA-1–/– mice as compared with age-matched control littermates in both CD4 and CD8 T cell populations (Fig. 2A). Expression of CD69 also tended to be increased but was more variable (data not shown). The significant increase in CD44high T cells was evident at 9–12 months in SPA-1–/– mice, whereas it was observed only at 16 months in the control littermates conforming to the previous report (20) (Fig. 2B). The reduction of Con A response was correlated with the increase in CD44high T cell population (Fig. 2B), implying selective unresponsiveness in the SPA-1–/– CD44high T cells. To directly investigate the possibility, we separated the T cells from 10-month-old SPA-1–/– and SPA-1++/+ mice into CD44high and CD44low populations and examined their Con A responsiveness in the presence of irradiated wild-type splenic adherent cells as Con A-presenting cells. As shown in Fig. 2C, SPA-1–/– CD44high T cells showed markedly impaired Con A response, whereas SPA-1–/– CD44low T cells did respond to Con A. In SPA-1+/+ mice, both CD44high and CD44low T cells responded comparably to Con A (Fig. 2C). Also, only SPA-1–/– CD44high T cells showed reduced proliferative response to PMA plus ionomycine (data not shown). The results suggested strongly that the age-dependent reduction of T cell responsiveness in SPA-1–/– mice was attributed to the progressive increase in TCR-unresponsive CD44high T cells.
Fig. 2.
Age-dependent increase in the anergic CD44high T cells in SPA-1–/– mice. (A) Representative two-color FACS profiles for CD4 or CD8 vs. CD44 of the 10-month-old SPA-1+/+ and SPA-1–/– spleen cells. (B Left) Proportions of CD44high T in the total CD3+ spleen cells from SPA-1+/+ (open columns) and SPA-1–/– (filled columns) mice of varying ages. The means and SE of the indicated numbers of mice are shown. *, P < 0.05. (Right) Maximal Con A responses of SPA-1+/+ (○) and SPA-1–/– (•) mice were plotted against the proportions of CD44high T cells. (C) Sorted CD44low (○) and CD44high (•) T cells from SPA-1+/+ and SPA-1–/– mice were stimulated with varying concentrations of Con A in the presence of the irradiated wild-type splenic adherent cells. The means and SE of triplicate cultures are shown.
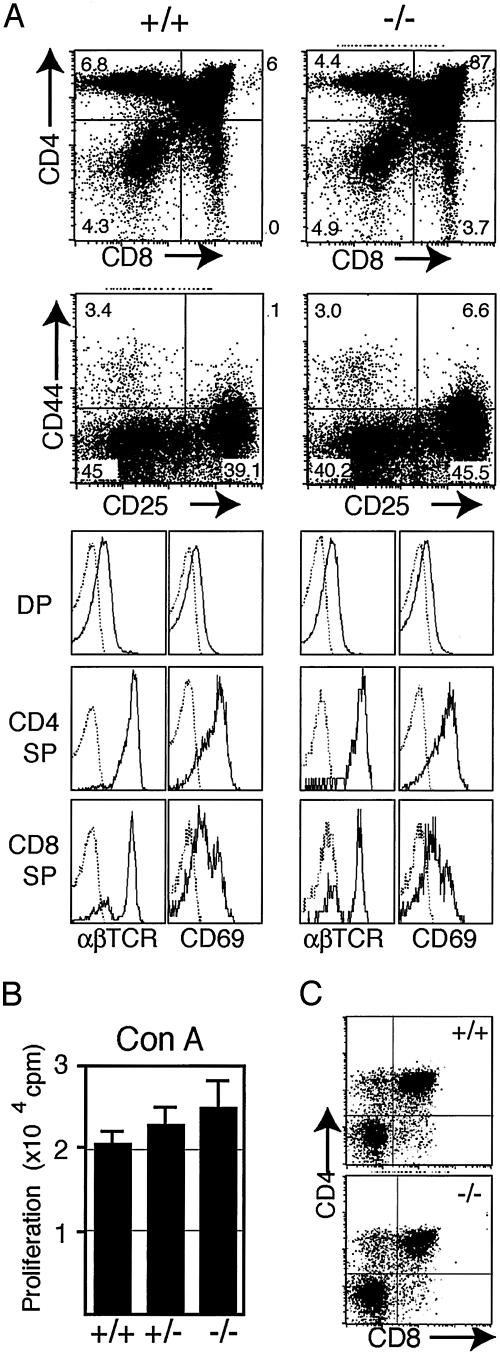
No Evidence for Abnormal T Cell Differentiation in SPA-1–/– Mice. Although CD44high T cells in normal mice represent the activated or memory T cells (21), an alternative possibility remained that SPA-1–/– CD44high T cells developed by altered differentiation in the thymus. To examine the possibility, we analyzed the thymus of SPA-1–/– mice. CD44 expressed at the earliest stages (DN I and II stages) was down-regulated at later stages in SPA-1–/– mice similar to the control mice, and differentiation profiles of double-positive (DP) and single-positive (SP) thymocytes were unchanged (Fig. 3A). Expression profiles of CD5 (data not shown) and CD69 (Fig. 3A) in the DP and SP populations were also normal, suggesting grossly normal positive selection. Essentially identical results were obtained in 3- and 8-month-old mice. Thymocytes from SPA-1–/– and SPA-1+/– mice responded to Con A comparably to those from SPA-1+/+ littermates (Fig., 3B). Because SPA-1–/– mice showed abnormal hematopoietic progenitors in BM (18), we further examined the differentiation of BM progenitors in thymic organ cultures. As shown in Fig. 3C, sorted lin– c-kit+ Sca-1high BM cells from 8-month-old SPA-1–/– mice showed indistinguishable T cell differentiation from those of control mice. Thus, it was suggested that the increased CD44high T cell population in SPA-1–/– mice represented the antigen-primed T cells as in normal mice.
Fig. 3.
Normal T cell differentiation in SPA-1–/– mice. (A) Thymocytes from 4-month-old SPA-1–/– and SPA-1+/+ mice were two-color analyzed for CD4 vs. CD8 and CD44 vs. CD25 (Left) or three-color analyzed for CD4, CD8, and αβTCR or CD69 (Right). (B) Thymocytes from 4-month-old SPA-1+/+, SPA-1+/–, and SPA-1–/– mice were stimulated with Con A for 2 days, and the proliferation was assayed. The means and SE of five mice for each group are shown. (C) Sorted lin– c-kit+ Sca-1high cells of 8-month-old SPA-1–/– and SPA-1+/+ BM were cultured with deoxyguanosine-treated fetal thymic lobes (100 cells per lobe) for 3 weeks.
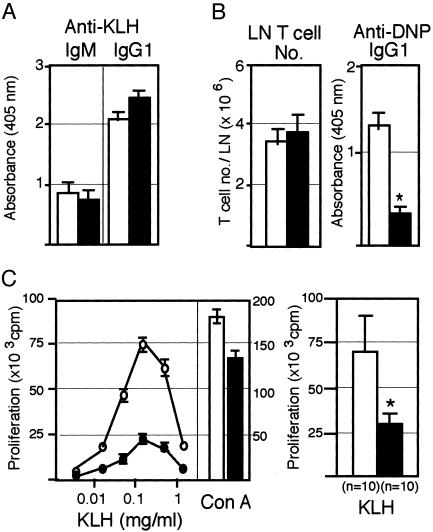
Selective Defect in the Specific Recall T Cell Response in SPA-1–/– Mice. To investigate the effects of antigen priming on the T cell responsiveness in vivo, we immunized 4-month-old SPA-1–/– mice with a T-dependent antigen KLH. SPA-1–/– mice showed the primary anti-KLH antibody response in vivo comparable to the control littermates (Fig. 4A). However, when regional lymph node (LN) cells of the immunized mice were challenged with TNP-KLH in vitro, anti-DNP IgG response was reduced significantly in SPA-1–/– mice despite the comparable T cell numbers in the regional LN (Fig. 4B). Considering that SPA-1–/– B cells produced anti-KLH IgG normally, the results suggested that the activation of KLH-primed helper T cells in response to TNP-KLH was impaired. This was confirmed by significant reduction in the proliferation of regional LN T cells from SPA-1–/– mice in response to KLH as compared with those from the control mice (Fig. 4C). Con A response of the same SPA-1–/– LN T cells was reduced only marginally, if any. These results suggested that antigenic stimulation of the naïve SPA-1–/– T cells in vivo was followed ultimately by the anergic state, resulting in the selective defect of antigen-specific T cell recall response.
Fig. 4.
Selective reduction of specific recall T cell responses to a T-dependent antigen. (A and B) Four-month-old SPA-1+/+ (open columns) and SPA-1–/– (solid columns) mice were immunized with KLH (100 μg) in CFA. Five weeks later, anti-KLH IgM and IgG1 in the sera (A) as well as anti-2,4-dinitrophenyl IgG in the supernatants of regional LN cells cultured in the presence of TNP-KLH (100 μg/ml) for 4 days (B) were determined with ELISA; the means and SE of 10 mice are indicated. T cell numbers in the regional LN are also indicated. *, P < 0.01. (C) Proliferation of the regional LN cells in response to the challenge with KLH or Con A. Representative dose–response profiles (Left) as well as the means and SE of maximal responses of 10 SPA-1+/+ and SPA-1–/– mice (Right) are shown. +/+: ○, open column; –/–: •, filled column. *, P < 0.01.
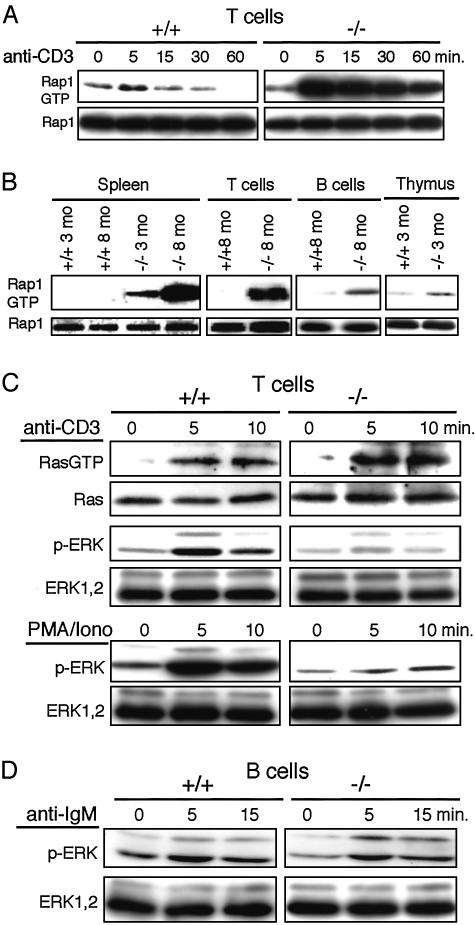
Age-Dependent Accumulation of Rap1GTP and Uncoupling of Ras-Mediated ERK Activation via TCR Stimulation in SPA-1–/– T Cells. We finally examined the Rap1 activation in SPA-1–/– T cells. T cells from the control mice showed transient Rap1 activation with anti-CD3 stimulation, which rapidly subsided within 15 min (Fig. 5A). In contrast, T cells from 2-month-old SPA-1–/– mice exhibited by far stronger Rap1 activation, which persisted more than 1 h (Fig. 5A), confirming that endogenous SPA-1 played a principal role in the down-regulation of Rap1 activation in T cells. Furthermore, freshly isolated T cells from 8-month-old SPA-1–/– mice exhibited marked accumulation of Rap1GTP, whereas those from age-matched control mice did not (Fig. 5B). B cells and thymocytes from SPA-1–/– mice showed only marginal Rap1GTP, consistent with redundant expression of rapGAP (see Fig. 1 A). Expression level of TCR/CD3 in T cells from the aged (10-month-old) mice was unchanged, and TCR internalization (data not shown) as well as Ras activation in response to CD3 stimulation occurred comparably or even better than those from the age-matched control mice (Fig. 5C). Nonetheless, these SPA-1–/– T cells exhibited little phosphorylation of ERK, whereas those from the control littermates showed significant ERK activation (Fig. 5C). ERK activation in response to PMA/ionomysin in the SPA-1–/– T cells was also reduced markedly (Fig. 5C). Although not shown, T cells from 3-month-old SPA-1+–/– mice showed comparable ERK activation to the control T cells by the same stimulation. In contrast, B cells from the 10-month-old SPA-1–/– mice showed comparable ERK activation in response to anti-IgM antibody to the control (Fig. 5D). These results suggested that the excess accumulation of endogenous Rap1GTP in the antigen-primed T cells from SPA-1–/– mice resulted in the interference with Ras-mediated ERK activation in response to the subsequent TCR stimulation.
Fig. 5.
Rap1GTP accumulation in SPA-1–/– T cells and uncoupling of Ras-mediated ERK activation via TCR stimulation. (A) T cells from 2-month-old SPA-1+/+ or SPA-1–/– mice were stimulated with CD3 cross-linking for varying periods, and Rap1 activation was assessed. (B) Rap1GTP in the fresh various lymphoid cells from 3- and 8-month-old SPA-1–/– and SPA-1+/+ mice were detected. (C) T cells from 10-month-old SPA-1–/– and SPA-1+/+ mice were stimulated with CD3 cross-linking or PMA/ionomycin for varying periods, and Ras and ERK activation was detected. (D) B cells from 10-month-old SPA-1–/– and SPA-1+/+ mice were stimulated with anti-IgM for varying periods, and ERK activation was detected.
Discussion
Rap1 GTPase is activated by antigen-receptor stimulation in lymphocytes, and recent studies suggested the significant roles of Rap1 signaling in the activation and fate decision of T cells after antigen stimulation (12–14). In most of these studies, a RapV12 mutant, which had been reported to be resistant to Rap1GAP (22) and considered as a canonical constitutive active form in an analogy with RasV12, was used. Recent study, however, demonstrated that GTPase activity of RapV12 was susceptible significantly to Rap1GAP, albeit much less than wild-type Rap1 (23), and thus the effects of “dominant active” function of RapV12 may have to be evaluated cautiously in the cells containing abundant Rap1GAPs. Rap1 GAPs play a major role in controlling the magnitude, duration, as well as localization of Rap1 activation in the cells (24). In the present study, to explore the roles of endogenous Rap1signaling in the immune responses, we have investigated the T cell functions of SPA-1–/– mice, taking an advantage that SPA-1 is a nonredundant Rap1 GAP in the peripheral T cells.
T cell differentiation in the thymus as well as number of peripheral T cells was apparently normal in SPA-1–/– mice. Nonetheless, the T cell responsiveness to Con A in terms of both proliferation and IL-2 production was reduced progressively in an age-dependent manner from around half a year, and it was attributed to the accelerated increase in CD44high T cell population that was unresponsive to Con A. In normal mice, CD44high T cells represent those that have been exposed to and activated by antigens in vivo, and at least a part of them comprises long-lived memory T cells (21). Thus, it was suggested that SPA-1–/– T cells primed by antigens in vivo were rendered anergic to the subsequent TCR stimulation at some point following the primary antigen activation. To support this, even young SPA-1–/– mice immunized with a T-dependent antigen KLH in vivo showed significantly reduced specific recall T cell responses to KLH in terms of both proliferation and carrier-specific helper effect on B cells, whereas the primary antibody response was unaffected. The results suggested that the age-dependent progressive reduction of T cell responsiveness in SPA-1–/– mice was antigen-driven in vivo, reminiscent of antigen-induced T cell anergy (3, 4). Unlike classical T cell anergy, however, it was suggested that the unresponsiveness followed the productive activation of naïve T cells in SPA-1–/– mice.
In parallel with the increase in anergic CD44high T cells in SPA-1–/– mice as they aged, progressive accumulation of intracellular Rap1GTP in the T cells was observed. Because the T cells from younger SPA-1–/– mice exhibited greater and by far more persistent Rap1 activation following TCR stimulation than control T cells, it was suggested that Rap1GTP accumulation reflected the sustained Rap1 activation in the antigen-primed T cells in SPA-1–/– mice, although it remained to be seen how long Rap1GTP persisted in the individual SPA-1–/– T cells following antigen stimulation in vivo. It might be also possible that suboptimal stimulations other than TCR that were involved normally in the maintenance of memory T cells such as MHC independent of peptides or cytokines contributed to the sustained Rap1 activation in the primed SPA-1–/– T cells. These T cells exhibited no detectable ERK activation in response to CD3 stimulation in vitro despite the normal or even enhanced Ras activation. The results strongly suggested that the preexisting Rap1GTP over certain levels in the T cells interfered with Ras-mediated ERK activation via TCR stimulation with the TCR-signaling upstream of Ras activation being unaffected. Although effects of excess Rap1GTP on other signaling pathways remain to be investigated, both compromised proliferation and IL-2 production via TCR in SPA-1–/– T cells are explained by the interference with Ras-mediated ERK activation (25). This is consistent with the reduced ERK activation and compromised proliferation of SPA-1–/– CD44high T cells by PMA plus ionomycin, which activate Ras via RasGRP (CalDAG-GEF II) (26). The results also confirmed that the unresponsiveness of SPA-1+–/– T cells was autonomous, as the response to PMA plus ionomycin required no interaction with accessory cells. The reasons for accelerated increase in CD44high T cells remained to be investigated. IL-2 production in response to Con A tended to be rather enhanced in the SPA-1–/– T cells at 3 months old, and thus it might reflect the enhanced primary activation of naïve T cells per se in vivo as indicated by a recent report (14).
Rap1 was isolated originally as a potential antagonist of oncogenic Ras (27), and the inhibitory effect of Rap1 signaling on Ras-ERK pathway was reported in some, but not all, cell line models in vitro (28–30). It was shown recently that the thymocytes of RapV12 transgenic mice under a CD2 promoter exhibited unaltered ERK activation in response to TCR stimulation (14). The amounts of RapV12GTP in the thymocytes of these transgenic mice were reported to be comparable to those in activated control thymocytes. On the other hand, present results indicated that Rap1GTP levels in the T cells of aged SPA-1–/– mice were much greater than those attained in the activated control T cells, strongly suggesting that the TCR-mediated Rap1 activation was under the profound regulation by endogenous SPA-1 in normal T cells. The counteracting effect of Rap1GTP on Ras-ERK signaling is suggested to be due to the competitive interference with RasGTP for c-Raf1 binding (31), and thus we speculate that the discrepancy may be in part due to the different amounts of Rap1GTP accumulation in the activated T cells. Present results strongly suggest that Rap1 may contribute to the setting of threshold for activation of antigen-primed T cells in response to the secondary stimulation via modulating Ras-ERK signaling in addition to the initiation of immunological synapse via integrin activation at the early phase of T cell activation (12). Efficient down-regulation of Rap1GTP by SPA-1 in T cells following the primary antigen activation is apparently crucial for them to return to the accessible state for the subsequent antigenic stimulation as functional memory cells.
SPA-1–/– mice developed myeloproliferative disorders due to the accumulation of Rap1GTP in the multipotential hematopoietic progenitors (18). Unlike in the T cells, Rap1GTP accumulation in the hematopoietic progenitors was associated with enhanced ERK activation, reinforcing that the effect of Rap1on ERK activation was cell-context-dependent (10, 32). Whereas the majority of them developed blast crisis of various lineages including myeloid, erythroid, and B cell lineages, no T celllineage blast crisis was observed (18). Similar observation was reported in the mouse model for AML1-ETO human leukemic oncogenes affecting hematopoietic progenitors (33). Progression of T cell dysfunction in SPA-1–/– mice preceded the development of leukemia, eliminating the possibility that the former was a secondary effect of myeloid malignancy. Rather, transfer of the BMC from preleukemic SPA-1–/– mice into SCID mice rapidly caused lethal leukemia (unpublished results). There is evidence that immune functions play significant roles in the control of myeloid malignancy both in human and mice (34, 35). Present results may suggest that age-dependent deterioration of T cell functions in part contributes to the development of myeloid leukemia of late onset in SPA-1–/– mice, providing an excellent model for understanding the mechanisms of immunological surveillance against malignancy.
Acknowledgments
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Culture, Sports, and Technology of Japan.
Abbreviations: GAP, GTPase-activating protein; ERK, extracellular signal-regulated kinase; TCR, T cell receptor; PMA, phorbol 12-myristate 13-acetate; KLH, keyhole limpet hemocyanin; TNP, 2,4,6-trinitrophenyl.
References
- 1.Dustin, M. L., Olszowy, M. W., Holdorf, A. D., Li, J., Bromley, S., Desai, N., Widder, P., Rosenberger, F., van der Merw, P. A., Allen, P. & Shaw, A. S. (1998) Cell 94, 667–677. [DOI] [PubMed] [Google Scholar]
- 2.Dustin, M. L. & Shaw, A. S. (1999) Science 283, 649–650. [DOI] [PubMed] [Google Scholar]
- 3.Powell, J. D., Ragheb, J. A., Kitagawa-Sakakida, S. & Schwartz, R. H. (1998) Immunol. Rev. 165, 287–300. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins, M. K. & Schwartz, R. H. (1987) J. Exp. Med. 165, 302–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wulfing, C., Sjaastad, M. D. & Davis, M. M. (1998) Proc. Natl. Acad. Sci. USA 95, 6302–6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dustin, M. L. & Cooper, J. A. (2000) Nat. Immunol. 1, 23–29. [DOI] [PubMed] [Google Scholar]
- 7.Tsukamoto, N., Hattori, M., Yang, H., Bos, J. L. & Minato, N. (1999) J. Biol. Chem. 274, 18463–18469. [DOI] [PubMed] [Google Scholar]
- 8.Katagiri, K., Hattori, M., Minato, N., Irie, S., Takatsu, K. & Kinashi, T. (2000) Mol. Cell. Biol. 20, 1956–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reedquist, K. A., Ross, E., Koop, E. A., Wolthuis, R. M., Zwartkruis, F. J., van Kooyk, Y., Salmon, M., Buckley, C. D. & Bos, J. L. (2000) J. Cell Biol. 148, 1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bos, J. L., de Rooij, J. & Reedquist, K. A. (2001) Nat. Rev. Mol. Cell Biol. 2, 369–377. [DOI] [PubMed] [Google Scholar]
- 11.Reedquist, K. A. & Bos, J. L. (1998) J. Biol. Chem. 273, 4944–4949. [DOI] [PubMed] [Google Scholar]
- 12.Katagiri, K., Hattori, M., Minato, N. & Kinashi, T. (2002) Mol. Cell. Biol. 22, 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boussiotis, V. A., Freeman, G. J., Berezovskaya, A., Barber, D. L. & Nadler, L. M. (1997) Science 278, 124–128. [DOI] [PubMed] [Google Scholar]
- 14.Sebzda, E., Bracke, M., Tugal, T., Hogg, N. & Cantrell, D. A. (2002) Nat. Immunol. 3, 251–258. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki, N., Ohba, Y., Kiyokawa, E., Kurata, T., Murakami, T., Ozaki, T., Kitabatake, A., Nagashima, K. & Matsuda, M. (1999) Nature 400, 891–894. [DOI] [PubMed] [Google Scholar]
- 16.Hattori, M., Tsukamoto, N., Nur-e-Kamal, M. S., Rubinfeld, B., Iwai, K., Kubota, H., Maruta, H. & Minato, N. (1995) Mol. Cell. Biol. 15, 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurachi, H., Wada, Y., Tsukamoto, N., Maeda, M., Kubota, H., Hattori, M., Iwai, K. & Minato, N. (1997) J. Biol. Chem. 272, 28081–28088. [DOI] [PubMed] [Google Scholar]
- 18.Ishida, D., Kometani, K., Yang, H., Kakugawa, K., Masuda, K., Iwai, K., Suzuki, M., Itohara, S., Nakahata, T., Hiai, H., et al. (2003) Cancer Cell 4, 55–65. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto, H., Ikawa, T., Ohmura, K., Fujimoto, S. & Katsura, Y. (2000) Immunity 12, 441–450. [DOI] [PubMed] [Google Scholar]
- 20.Miller, R. A. (1996) Science 273, 70–74. [DOI] [PubMed] [Google Scholar]
- 21.Sprent, J. & Tough, D. F. (2001) Science 293, 245–248. [DOI] [PubMed] [Google Scholar]
- 22.Maruta, H., Holden, J., Sizeland, A. & D'Abaco, G. (1991) J. Biol. Chem. 266, 11661–11668. [PubMed] [Google Scholar]
- 23.Brinkmann, T., Daumke, O., Herbrand, U., Kuhlmann, D., Stege, P., Ahmadian, M. R. & Wittinghofer, A. (2002) J. Biol. Chem. 277, 12525–12531. [DOI] [PubMed] [Google Scholar]
- 24.Ohba, Y., Kurokawa, K. & Matsuda, M. (2003) EMBO J. 22, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayter, S. I., Woodrow, M., Lucas, S. C., Cantrell, D. A. & Downward, J. (1992) EMBO J. 11, 4549–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebinu, J. O., Bottorff, D. A., Chan, E. Y., Stang, S. L., Dunn, R. J. & Stone, J. C. (1998) Science 280, 1082–1086. [DOI] [PubMed] [Google Scholar]
- 27.Kitayama, H., Sugimoto, Y., Matsuzaki, T., Ikawa, Y. & Noda, M. (1989) Cell 56, 77–84. [DOI] [PubMed] [Google Scholar]
- 28.Cook, S. J., Rubinfeld, B., Albert, I. & McCormick, F. (1993) EMBO J. 12, 3475–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu, C. D., Kariya, K., Kotani, G., Shirouzu, M., Yokoyama, S. & Kataoka, T. (1997) J. Biol. Chem. 272, 11702–11705. [DOI] [PubMed] [Google Scholar]
- 30.Zwartkruis, F. J., Wolthuis, R., M., Nabben, N., M., Franke, B. & Bos, J. L. (1998) EMBO J. 17, 5905–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada, T., Hu, C. D., Jin, T. G., Kariya, K., Yamawaki-Kataoka, Y. & Kataoka, T. (1999) Mol. Cell. Biol. 19, 6057–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.York, R. D., Yao, H., Dillon, T., Ellig, C. L., Eckert, S. P., McCleskey, E. W. & Stork, P. J. (1998) Nature 392, 622–626. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi, M., O'Brien, D., Kumaravelu, P., Lenny, N., Yeoh, E. J. & Downing, J. R. (2002) Cancer Cell 1, 63–74. [DOI] [PubMed] [Google Scholar]
- 34.Pear, W. S., Miller, J. P., Xu, L., Pui, J. C., Soffer, B., Quackenbush, R. C., Pendergast, A. M., Bronson, R., Aster, J. C., Scott, M. L. & Baltimore, D. (1998) Blood 92, 3780–3792. [PubMed] [Google Scholar]
- 35.Kolb, H. J., Schattenberg, A., Goldman, J. M., Hertenstein, B., Jacobsen, N., Arcese, W., Ljungman, P., Ferrant, A., Verdonck, L., Niederwieser, D., et al. (1995) Blood 86, 2041–2050. [PubMed] [Google Scholar]