Abstract
In addition to inhibiting cyclooxygenase (COX)-1-derived prostanoid biosynthesis, aspirin acetylates COX-2, enabling the conversion of arachidonic acid to 15(R)-epi lipoxin A4, or aspirin-triggered lipoxin (ATL). Selective COX-2 inhibitors block ATL formation and exacerbate mucosal injury in rats treated with aspirin. In the present study, we have examined whether inhibition of COX-2 activity in healthy volunteers taking aspirin exacerbates gastric mucosal injury and if such an effect would be prevented by NCX-4016, a NO-releasing derivative of aspirin. Thirty-two volunteers were randomized to receive 2 wk of treatment with NCX-4016 (800 mg twice a day) or aspirin (100 mg once a day) alone or in combination with 200 mg of celecoxib twice a day. Mucosal damage was assessed by endoscopy. The mean mucosal injury score was 5.8 ± 1.8 in subjects treated with aspirin and 2.4 ± 0.7 (P < 0.01 vs. aspirin) in subjects treated with NCX-4016. Administration of celecoxib increased the injury score in volunteers treated with aspirin (9.9 ± 1.9) but not in subjects taking NCX-4016 (1.5 ± 0.8). Aspirin and NCX-4016 caused a comparable suppression of serum thromboxane B2 levels and increased urinary excretion of ATL. Celecoxib inhibited endotoxin-induced prostaglandin E2 generation in whole blood by ≈80% and abolished ATL formation. These findings suggests that (i) aspirin and NCX-4016 trigger ATL formation in humans, (ii) celecoxib inhibits ATL formation and exacerbates the mucosal injury caused by low doses of aspirin, and (iii) the NO-donating moiety of NCX-4016 protects the gastric mucosa even in the presence of suppression of COX-1 and COX-2.
Prostaglandins (PGs) are lipid mediators of importance in physiological responses, inflammation, and thrombosis (1). They are formed from arachidonic acid by the catalytic activity of cyclooxygenase (COX) (2–4). It is now recognized that there are at least two related but distinct gene products that possess COX activity, COX-1 and COX-2 (2–4). COX-1 is expressed constitutively in most tissues and is involved in “housekeeping” functions, such as the maintenance of gastrointestinal integrity and vascular homeostasis (1, 2). COX-2 is induced as an intermediate-early gene in a more limited repertoire of cells, notably monocytes, macrophages, neutrophils, and endothelial cells, in response to bacterial endotoxin, various growth factors, and cytokines (2–5). Although these observations have led to the hypothesis that COX-2 expression mediates the enhanced prostanoid release, which characterizes inflammatory responses, COX-2-derived eicosanoids are increasingly recognized as physiologically important mediators of gastrointestinal, cardiovascular, and renal homeostasis (2, 5).
The use of aspirin has increased considerably since it was shown to reduce the risk of myocardial infarction and stroke (6). Aspirin imparts its antiplatelet activity by irreversibly acetylating COX-1, leading to inhibition of platelet thromboxane (TX) A2 formation (1, 7). In contrast to nonsteroidal antiinflammatory drugs (NSAIDs) and selective COX-2 inhibitors, aspirin restricts access of arachidonic acid (AA) to the COX catalytic core by covalently modifying a serine residue near the active site of the enzyme (1, 7). However, whereas acetylation of residue 529 of COX-1 abolishes the enzyme's capacity to oxidize AA, the acetylation of the corresponding serine residue in COX-2 (Ser-516) modifies that enzyme such that it performs an incomplete reaction in which AA is converted to 15-hydroxyeicosatetraenoic acid, carrying its C15 alcohol in the R configuration [15(R)-hydroxyeicosatetraenoic acid] (8–10). 15(R)-hydroxyeicosatetraenoic acid is converted by lipoxygenase isoforms to 15(R)-epi-lipoxin A4 or aspirin-triggered lipoxin (ATL). This lipid mediator is generated through a transcellular route at the leukocyte–endothelial cell interface and functions as a braking signal limiting neutrophil recruitment to sites of inflammation (9). A growing body of evidence supports the notion that locally generated ATL limits leukocyte recruitment in the gastric microcirculation (11, 12), an event that is thought to play a critical role in the pathogenesis of aspirin-induced gastric injury. Consistent with this view, COX-2 inhibition suppresses ATL formation and exacerbates aspirin-induced gastric damage in rats (11, 12).
Unlike aspirin, NSAIDs bind reversibly to COX, depressing platelet function for only a limited amount of time (6), whereas selective COX-2 inhibitors do not interfere with platelet function at all (13). The use of selective COX-2 inhibitors has been linked to an increased risk of nonfatal myocardial events (14), raising the question of which approach to cardioprotection should be taken in patients with cardiovascular risk who are prescribed a selective COX-2 inhibitor. Because of the lack of inhibitory effect of selective COX-2 inhibitors on platelet aggregation, coadministration of low doses of aspirin has been recommended (13, 14). However, whether this combination maintains any advantage of a selective COX-2 inhibitor over a nonselective NSAID with respect to gastrointestinal side effects remains to be tested (14, 15).
NCX-4016 is a NO-releasing derivative of aspirin (16–21). Both the aspirin and the NO-releasing moieties of this compound contribute to its effectiveness (16). Thus, not only does NCX-4016 inhibit COX-1 and COX-2 activity in vivo (16–18) and in vitro (19), it also increases platelet cyclic guanosyl monophate concentrations (17), inhibits platelet aggregation induced by adenosine diphosphate and thrombin (i.e., aspirin-resistant aggregation) (16), and modulates tissue factor expression and activity in vivo and in vitro (19). Consistent with the fact that NO is a multifunctional regulatory molecule and mediates many components of gastrointestinal mucosal defence (20), NCX-4016 spares the gastrointestinal tract in healthy human volunteers (21) while retaining antiplatelet activity. Whether or not NCX-4016 triggers ATL formation and spares the gastric mucosa when administered in combination with a selective COX-2 inhibitor in humans is unknown.
Materials and Methods
The primary aim of this study was to assess the gastrointestinal safety of coadministering NCX-4016 or aspirin together with celecoxib in a randomized, blind, parallel-group endoscopic study. The protocol study was carried out by using commercially available aspirin and celecoxib, and the endoscopists (S.F., L.S., and A.M.) who performed the study were unaware of the treatment that the participants had received. The protocol was approved by an institutional ethics committee and the study was carried out at the facilities of Contract Research Organization-Alliance (Arzo, Switzerland). All subjects gave written, informed consent before entering the study.
Subjects and Study Design. Thirty-two healthy subjects (23 male and 9 female) with ages ranging from 19 to 42, were randomized to receive one of the following treatments for 14 days: NCX-4016, 800 mg twice a day (b.i.d.); NCX-4016, 800 mg b.i.d. plus celecoxib 200 mg b.i.d.; aspirin, 100 mg once daily; and aspirin, 100 mg once daily plus celecoxib 200 mg b.i.d. These drug regimens were chosen on the following assumptions: (i) the dose of aspirin is that currently recommended for primary and secondary prevention of cardiovascular events and fully inhibits platelet aggregation and TXA2 formation (6); (ii) the dose of celecoxib used is that currently recommended for treating pain and inflammation in rheumatoid arthritis and osteoarthritis patients (13, 14); and (iii) the dose of NCX-4016 was chosen on the basis of a previous study demonstrating that it fully inhibits platelet aggregation and TXA2 formation (21) while sparing the gastric mucosa in humans. The medications were taken orally at 8 a.m. and 8 p.m. at the clinical site for 7 consecutive days. Blood samples for basal, intermediate (day 8), and posttreatment hematobiochemical analyses, platelet aggregation studies, serum TXB2, and plasma celecoxib and salicylate levels were collected 3 h after drug administration. The last dose of the study medications was taken the evening before the second (posttreatment) endoscopy. The use of any drug not included in the study was prohibited. A serum sample was obtained from each subject and analyzed for the presence of IgG Abs to Helicobacter pylori (EIAgen kit, Biochem Immunosystem, Bologna, Italy) (21). The sensitivity and specificity of the test, as reported by the manufacturer, were 95.7% and 98.4%, respectively.
Endoscopy. After an overnight fast, the endoscopic examination was performed by using an Olympus Exera GIF-Q160 (Melville, NY), with premedication with 3–5 mg of i.v. midazolam (Dormicum Roche, Hoffmann–La Roche, Basel). All subjects received topical lidocaine 2% (Xylocayne, AstraZeneca, London) immediately before endoscopic intubation. The entire stomach and duodenum were systematically examined from the fundus to the duodenum in a proximal to distal manner to minimize errors that might result from a misinterpretation of mucosal damage caused by the passage of the instrument. Each endoscopic procedure was completely recorded with a video recorder. Hemorrhagic and erosive mucosal lesions were graded on a 0–4 scale by using the following criteria: grade 0 = normal mucosa; grade 1 = 1–3 erosions or submucosal hemorrhages; grade 2 = 4–10 erosions or submucosal hemorrhages; grade 3 = >10 erosions or submucosal hemorrhages; grade 4 = ulcer or diffuse submucosal hemorrhages (21). Separate endoscopic injury scores for hemorrhagic and erosive lesions were assigned for the gastric fundus, gastric body, gastric antrum, and duodenum. Erosions and ulcers were defined as white-based, well circumscribed mucosal breaks and were measured by close apposition of an endoscopic forcep with defined dimensions. An erosion was defined as a flat lesion with no discernible depth; an ulcer was defined as a mucosal break ≥3 mm in diameter with unequivocal depth (21).
Plasma TXB2 and Urinary 11-Dehydro(dh)-TXB2. For the measurement of serum TXB2, blood samples were taken in the morning at the beginning of the study, on day 8, and at the end of the study (day 15) 6 h after drug administration. Each blood sample was drawn by a 21-gauge cannula inserted into the antecubital vein. Nonanticoagulated blood samples were collected in a dry syringe and immediately transferred into a glass tube. The samples were allowed to clot for 1 h at 37°C and then centrifuged at 2,000 × g for 15 min (22). The supernatant serum was recovered and frozen at –20°C until assayed. Serum TXB2 concentrations were measured by using a specific ELISA (Cayman Chemical, Ann Arbor, MI) (21, 22). To determine the urinary excretion of 11-dh-TXB2, a urinary TXB2 metabolite, urine samples collected for 6 h after drug administration were subjected to purification procedures as described (21), and 11-dh-TXB2 was measured by a commercial ELISA kit (Cayman Chemical).
Ex Vivo Generation of PGE2. Aliquots of peripheral blood samples (1 ml) containing 10 units of sodium heparin were incubated in both the absence and the presence of 10 μg/ml endotoxin (lipopolysaccharide from Escherichia coli K-235, Sigma) for 24 h at 37°C as described (21, 22). Plasma was separated by centrifugation (10 min at 2,000 rpm) and kept at –80°C until assayed for PGE2. COX-2 activity was defined as the production of PGE2 in the endotoxin-treated blood over that of background levels in unstimulated blood at time 0. PGE2 concentrations in supernatants were measured by using commercial ELISA kits (Cayman Chemical).
ATL Assay. ATL concentrations in the urine were measured by using a commercial ELISA kit (Neogen, Lansing, MI) according to the manufacturer's instructions (11, 12). Urine was collected on days 0, 8, and 15 for 6 h after drug administration, and samples were extracted according to a published method (23). The Ab used in this assay specifically recognizes 15(R)-epi-LXA4 and has been characterized by others (10). The identity of 15(R)-epi-LXA4 in samples was also confirmed by using a dual pump RP-HPLC analysis (11, 23). The column (Waters Symmetry C18, 3 μm, 2.1 × 150 mm) was eluted with MeOH/H2O/acetic acid (65:35:0.01; vol/vol/vol) at a flow rate of 0.2 ml/min. Postrun analysis was performed with the millennium 32 chromatography manager (Waters). LXA4 and 15(R)-epi-LXA4 were identified by online UV spectral analysis and comparison of their retention times with those of authentic standards (LXA4 was obtained from Cascade Biochem, Reading, U.K.). The 15(R)-epi-LXA4-methylester (kindly provided by C. N. Serhan, Harvard Medical School, Boston) was converted to free acid by saponification as described (23).
Plasma Salicylate and Celecoxib Levels. For measurement of plasma salicylate and celecoxib levels, blood samples were taken before and 6 h after the last dose of each drug. Blood samples were centrifuged at 4,200 × g for 10 min at 4°C. Specimens were subsequently stored at –20°C and assayed by HPLC (Prostar 330, Varian) according to a published method (24). The celecoxib concentrations were determined by using a HPLC assay with fluorescence detection for human plasma as described (25).
Statistical Analysis. Data were analyzed by using a nonparametric ANOVA model for a 2 × 2 factorial design after transforming endoscopic scores into ranks according to the RT-1 transformation of Conover and Iman (26). Exact P values were obtained from exact permutation resampling (27) carried out by resorting to proc multitest, Version 8.2 (SAS Institute, Cary, NC). TXB2, PGE2, 11-dh-TXB2, and ATL were compared by using the above ANOVA model without rank transformation. Nonparametric statistics were applied when appropriate. Differences between treatment groups were considered significant with P values <0.05.
Results
Each drug regimen was well tolerated. No volunteer withdrew from the study or experienced serious adverse events. There were no clinically significant changes identified in hematology, biochemistry, urinalysis, supine vital signs, or physical examination during the course of the study period. Serum IgG Abs to H. pylori were found in 11 subjects: four of the volunteers treated with NCX-4016 alone, two of the volunteers treated with NCX-4016 in combination with celecoxib, two of the volunteers treated with aspirin alone, and three of the volunteers treated with NCX-4016 in combination with celecoxib (not significant among groups).
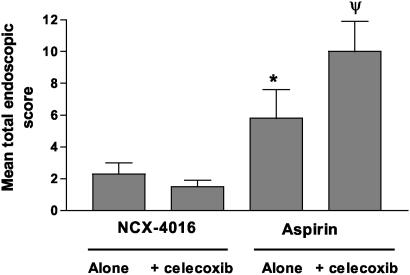
Celecoxib Exacerbates Gastrointestinal Damage Induced by Aspirin. Mean stomach or duodenum endoscopic scores at the first endoscopy ranged from 0 to 0.8. Administration of a morning dose of 100 mg of aspirin for 14 days (Fig. 1) resulted in mean total endoscopic score of 5.8 ± 1.8 (P < 0.001 vs. predose). Coadministration of celecoxib with aspirin exacerbated gastric damage caused by aspirin alone, resulting in a mean endoscopic score of 9.9 ± 1.9 (P < 0.05 vs. aspirin alone). In contrast to aspirin, twice-daily administration of NCX-4016 at 800 mg b.i.d. resulted in a mean total endoscopic score of 2.4 ± 0.7 (P < 0.05 vs. aspirin). Coadministration of celecoxib and NCX-4016 did not increase the extent of gastrointestinal damage over that of NCX-4016 alone, with a mean total endoscopic score of 1.5 ± 0.8 (P < 0.0001 vs. aspirin plus celecoxib; P > 0.05 vs. NCX-4016 alone) (Fig. 1).
Fig. 1.
Administration of celecoxib exacerbates gastric mucosal injury caused by aspirin but not by NCX-4016. Subjects (n = 8 per group) were treated with NCX-4016 (800 mg b.i.d.) alone or in combination with celecoxib (200 mg b.i.d.) or aspirin (100 mg/day) alone or in combination with celecoxib (200 mg b.i.d.) for 14 days. Upper gastrointestinal endoscopy was performed by a blind observer at the beginning and at the end of the study. *, P < 0.01 vs. NCX-4016 alone. Ψ, P < 0.05 vs. aspirin alone, and P < 0.0001 vs. NCX-4016 plus celecoxib.
Administration of aspirin or NCX-4016 alone or in combination with celecoxib did not differentially affect mucosal PGE2, as measured in gastric biopsies taken on day 14. The levels of mucosal PGE2 were: 147.1 ± 28.10, 147.0 ± 23.0, 153.0 ± 32.0, and 143.0 ± 41.0 pg/mg protein, respectively, in the subjects treated with NCX-4016 alone, NCX-4016 plus celecoxib, aspirin alone, and aspirin plus celecoxib. These values were not statistically different from one another.
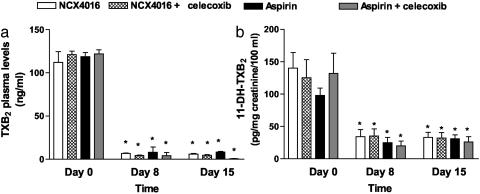
NCX-4016 and Aspirin Inhibit COX-1 Activity in Vivo. Serum TXB2 levels, a measure of platelet COX-1 activity, did not differ significantly among the groups at baseline. Administration of either NCX-4016 or aspirin (Fig. 2a) reduced these concentrations by ≈95% (P < 0.001 vs. baseline). NCX-4016 and aspirin also reduced urinary excretion of 11-dh-TXB2 to a comparable extent (Fig. 2b). In participants taking celecoxib in combination with NCX-4016 or aspirin, the patterns of serum TXB2 production and 11-dh-TXB2 excretion were similar to those in subjects taking NCX-4016 or aspirin alone (Fig. 2).
Fig. 2.
Inhibition of platelet COX-1 activity, as assessed by measurement of serum TXB2 (a) and urinary concentrations of 11-dh-TXB2 (b) on days 8 and 15 after treatment with NCX-4016 (800 mg b.i.d.) or aspirin (100 mg/day) alone or in combination with celecoxib (200 mg b.i.d.). *, P < 0.0001 vs. pretreatment.
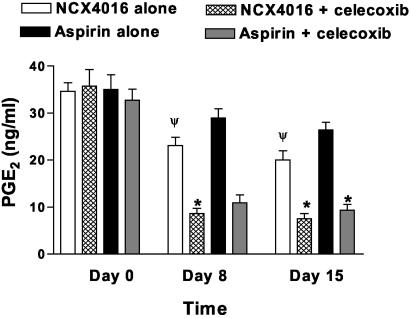
Celecoxib Inhibits COX-2 Activity ex Vivo. In the subjects taking NCX-4016, there was ≈40% inhibition of COX-2 activity (Fig. 3), as reflected by the level of endotoxin-stimulated PGE2 formation measured in blood samples taken 6 h after the administration of drug on days 8 and 15 (P < 0.001 in comparison with day 0). Inhibition of COX-2 activity by the combination of NCX-4016 and celecoxib (Fig. 3) approached 85% on days 8 and 15, an effect that is consistent with the ability of celecoxib to inhibit COX-2 activity (P < 0.0001 vs. day 0). PGE2 production was not inhibited by administration of low doses of aspirin (Fig. 3), but, again, addition of celecoxib to aspirin resulted in 80% inhibition of COX-2-derived prostanoid synthesis (P < 0.0001 in comparison with day 0).
Fig. 3.
Inhibition of COX-2 activity by celecoxib and NCX-4016, measured as whole-blood endotoxin-stimulated PGE2 synthesis. Ex vivo PGE2 generation in response to bacterial endotoxin was measured as described in Materials and Methods, before and on days 8 and 15. *, P < 0.0001 vs. pretreatment. Ψ, P < 0.05 vs. pretreatment.
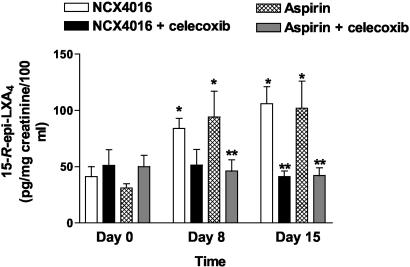
Celecoxib Inhibits ATL Formation Induced by Aspirin and NCX-4016. As shown in Fig. 4, urinary excretion of ATL on days 8 and 15 was significantly increased in participants who took NCX-4016 or aspirin (P < 0.01 in comparison with day 0). Administration of celecoxib almost completely inhibited ATL excretion (P < 0.01 vs. NCX-4016 and aspirin alone), confirming that ATL was generated through a COX-2-dependent pathway.
Fig. 4.
Effect of NCX-4016, aspirin, and celecoxib on urinary excretion of ATL. ATL generation was measured as described in Materials and Methods before treatment and on days 8 and 15 after treatment with NCX-4016 (800 mg b.i.d.) or aspirin (100 mg/day), alone or in combination with celecoxib (200 mg b.i.d.). *, P < 0.01 vs. pretreatment. **, P < 0.05 vs. NCX-4016 and aspirin.
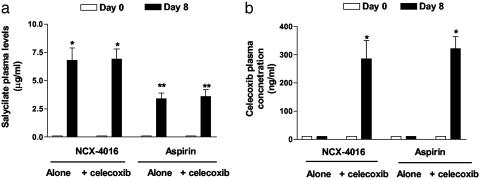
Salicylate and Celecoxib Plasma Levels. At baseline, plasma salicylate and celecoxib levels were undetectable in all volunteers (data not shown). On day 8, at steady state, salicylates were found in the plasma of all participants and were significantly higher in subjects taking NCX-4016 (P < 0.001, NCX-4016 vs. aspirin). On day 8, celecoxib was found only in the plasma of participants taking NCX-4016 or aspirin in combination with this drug (Fig. 5).
Fig. 5.
Effect of different drug regimens on plasma salicylate and celecoxib levels. Blood samples were taken 6 h after the morning dose of each drug on days 0, 8, and 15 (data not shown). *, P < 0.001 vs. baseline; **, P < 0.01 NCX-4016 vs. aspirin.
Discussion
The recognition of the failure of selective COX-2 inhibitors (13, 14) to afford cardioprotection has raised interest in their use in combination with aspirin. In the present study, we have examined the gastrointestinal consequences of inhibition of COX-2 with a therapeutically relevant dose of celecoxib in subjects taking low-dose aspirin. This drug regimen simulates, albeit in an acute setting, the clinical condition of patients taking low doses of aspirin in combination with celecoxib. The results of this investigation demonstrate that although celecoxib does not interfere with the antiplatelet activity of aspirin (i.e., suppression of TX synthesis), it almost doubled the gastrointestinal injury caused by aspirin, supporting the notion that simultaneous inhibition of COX-1 and COX-2 abrogates the gastric-sparing activity of celecoxib (5). Consistent with this view, animal studies have provided evidence that COX-1 and COX-2 mediate different protective functions in the gastric mucosa with COX-1 involved in regulating mucosal blood flow (5) and COX-2 regulating leukocyte adherence to endothelial cells (5), the latter being an event that is mechanistically involved in the pathogenesis of NSAID gastropathy (16).
The results of the Celecoxib Long-Term Arthritis Safety Study (CLASS) (15) trial have raised concerns that administration of celecoxib to arthritis patients taking low doses of aspirin, up to 325 mg/day, would increase the risk of gastrointestinal bleeding and/or perforation. Indeed, the results of this trial demonstrated that a 4-fold increase in the incidence of gastrointestinal bleeding occurred in a subgroup of patients taking celecoxib in combination with aspirin, suggesting that simultaneous inhibition of COX-1 and COX-2 might damage the gastric mucosa to the same extent as nonselective NSAIDs (15). We have now provided further evidence in support of this hypothesis by showing that in healthy human volunteers, celecoxib exacerbates injury caused by 100 mg/day aspirin. Although the relation of pilot endoscopic studies to gastrointestinal toxicity of NSAIDs in osteoarthritis or rheumatoid arthritis patients is imperfect, and assessment of gastrointestinal side effects of coadministering celecoxib with low-dose aspirin will require an appropriately designed outcome trial, the results of our study proves the concept that simultaneous inhibition of COX-1 and COX-2 with therapeutically relevant doses of selective inhibitors (aspirin and celecoxib) in humans might increase the tendency of aspirin to injure the gastrointestinal mucosa.
In the present study, we have also demonstrated that administration of NCX-4016 (16), at a dose that inhibits COX-1 and COX-2 activity, spares the gastric mucosa. Because administration of NCX-4016 resulted in mucosal concentrations of gastric PGE2 similar to those in subjects taking aspirin alone or in combination with celecoxib, and NCX-4016 fully inhibited TXB2 formation, the present results suggest that mechanisms other than gastric mucosal PG preservation are involved in gastric protection afforded by NCX-4016. Topical application of NSAIDs decreases gastric mucosal blood flow, an event that is believed to play a mechanistic role in the pathogenesis of the so-called NSAID gastropathy (5, 20). NCX-4016 not only protects against this detrimental effect but even increases gastric mucosal blood flow in rodents, an effect that is related to the local release of NO (5, 17, 20). Although preservation of gastric mucosal blood flow is an important mechanism, NO exerts a number of other protective activities, including inhibition of neutrophil recruitment and function (16) and protection of endothelial and gastric epithelial cells from apoptosis and necrosis (18, 20). Of relevance, NCX-4016 not only releases NO as conventional NO donors, although with a different kinetic pattern and mechanism, but its application to the gastrointestinal mucosa fully reproduces the effect of NO on mucosal blood flow (18, 19).
In this study, we have also provided evidence that administration of NCX-4016 and aspirin increases the urinary excretion of ATL and that this effect is abrogated by cotreating volunteers with celecoxib (8–12). ATL is a lipid mediator generated by aspirin-acetylated COX-2 (8). A growing body of evidence is now linking gastric ATL formation to the adaptation of the stomach to chronic aspirin ingestion (11, 12, 28). Because ATL functions as a braking signal, limiting neutrophil recruitment in the gastric microcirculation (11, 12, 28), its inhibition might well explain the exacerbation of gastric injury observed in subjects treated with the combination of aspirin plus celecoxib. Consistent with this view, we have detected ATL in the urine of subjects treated with aspirin or NCX-4016 but not in the urine of subjects taking celecoxib. Although this measure reflects the systemic effect of aspirin (23), this finding indicates that the aspirin-like moiety of NCX-4016 functions as an effective COX inhibitor and acetylates COX-2 in humans.
In summary, by demonstrating that aspirin and NCX-4016 increase ATL formation in healthy human volunteers, we have provided evidence that acetylated COX-2 might play a role in human disease. We have also shown that simultaneous administration of a low dose of aspirin in combination with celecoxib increases the tendency of aspirin to cause gastric injury. Finally, our results support the notion that NO released from the NO-donating moiety of NCX-4016 protects the gastric mucosa even in the absence PGE2 and ATL. These results are of clinical relevance and should be considered when prescribing selective COX-2 inhibitors to patients taking low doses of aspirin.
Acknowledgments
We thank Dr. Alberto Fransioli, Andrea Mencarelli, Barbara Federici, Cinzia Fanini, Dr. Silvana Farneti, Dr. Laura Sanpaolo, and Dr. Stefano Orlandi for assistance. J.L.W. is an Alberta Heritage Foundation for Medical Research Scientist whose work is supported by grants from the Canadian Institutes of Health Research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: COX, cyclooxygenase; ATL, aspirin-triggered lipoxin; b.i.d., twice a day; PG, prostaglandin; TX, thromboxane; 11-dh-TXB2, 11-dehydro-TXB2; NSAID, nonsteroidal antiinflammatory drug.
References
- 1.Smith, W. L., DeWitt, D. L. & Garavito, R. M. (2000) Annu. Rev. Biochem. 69, 149–182. [DOI] [PubMed] [Google Scholar]
- 2.Smith, W. L. & Langenbach, R. (2001) J. Clin. Invest. 107, 1491–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masferrer, J. L., Zweifel, B. S., Seibert, K. & Needleman, P. (1990) J. Clin. Invest. 86, 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie, W. L., Chipman, J. G., Robertson, D. L., Erikson, R. L. & Simmons, D. L. (1991) Proc. Natl. Acad. Sci. USA 88, 2692–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace, J. L., McKnight, W., Reuter, B. K. & Vergnolle, N. (2000) Gastroenterology 119, 706–714. [DOI] [PubMed] [Google Scholar]
- 6.Patrono, C. (1994) N. Engl. J. Med. 330, 1287–1294. [DOI] [PubMed] [Google Scholar]
- 7.Loll, P. J., Picot, D. & Garavito, R. M. (1995) Nat. Struct. Biol. 2, 637–643. [DOI] [PubMed] [Google Scholar]
- 8.Claria, J. & Serhan, C. N. (1995) Proc. Natl. Acad. Sci. USA 92, 9475–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan, C. N. & Oliw, E. (2001) J. Clin. Invest. 107, 1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang, N., Takano, T., Clish, C., Petasis, N., Tai, H. H. & Serhan, C. (1998) J. Pharmacol. Exp. Ther. 287, 779–790. [PubMed] [Google Scholar]
- 11.Fiorucci, S., de Lima, O. M., Jr., Mencarelli, A., Palazzotti, B., Distrutti, E., McKnight, W., Dicay, M., Ma, L., Romano, M., Morelli, A., et al. (2002) Gastroenterology 123, 1598–1606. [DOI] [PubMed] [Google Scholar]
- 12.Fiorucci, S., Distrutti, E., Menezes de Lima, O., Romano, M., Mencarelli, A., Barbanti, M., Palazzini, E., Morelli, A. & Wallace, J. L. (2003) FASEB J. 17, 1171–1173. [DOI] [PubMed] [Google Scholar]
- 13.FitzGerald, G. A. & Patrono, C. (2001) N. Engl. J. Med. 345, 433–442. [DOI] [PubMed] [Google Scholar]
- 14.Bombardier, C., Laine, L., Reicin, A., Shapiro, D., Burgos-Vargas, R., Davis, B., Day, R., Ferraz, M. B., Hawkey, C. J., Hochberg, M. C., et al. (2000) N. Engl. J. Med. 343, 1520–1528. [DOI] [PubMed] [Google Scholar]
- 15.Silverstein, F. E., Faich, G., Goldstein, J. L., Simon, L. S., Pincus, T., Whelton, A., Makuch, R., Eisen, G., Agrawal, N. M., Stenson, W. F., et al. (2000) J. Am. Med. Assoc. 284, 1247–1255. [DOI] [PubMed] [Google Scholar]
- 16.Wallace, J. L., Ignarro, L. J. & Fiorucci, S. (2002) Nat. Rev. Drug Discovery 1, 375–382. [DOI] [PubMed] [Google Scholar]
- 17.Wallace, J. L., McKnight, W., del Soldato, P., Baydoun, A. R. & Cirino, G. (1995) J. Clin. Invest. 96, 2711–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorucci, S., Antonelli, E., Santucci, L., Morelli, O., Miglietti, M., Federici, B., Mannucci, R., del Soldato, P. & Morelli, A. (1999) Gastroenterology 116, 1089–1106. [DOI] [PubMed] [Google Scholar]
- 19.Fiorucci, S., Mencarelli, A., Meneguzzi, A., Lechi, A., Morelli, A., del Soldato, P. & Minuz, P. (2002) Circulation 106, 3120–3125. [DOI] [PubMed] [Google Scholar]
- 20.Wallace, J. L. & Miller, M. J. (2000) Gastroenterology 119, 512–520. [DOI] [PubMed] [Google Scholar]
- 21.Fiorucci, S., Santucci, L., Gresele, P., Maffei-Faccino, R., del Soldato, P. & Morelli, A. (2003) Gastroenterology 124, 600–607. [DOI] [PubMed] [Google Scholar]
- 22.Patrignani, P., Panara, M. R., Greco, A., Fusco, O., Natoli, C., Iacobelli, S., Cipolline, F., Ganci, A., Creminon, C., Maclouf, J., et al. (1994) J. Pharmacol. Exp. Ther. 271, 1705–1712. [PubMed] [Google Scholar]
- 23.Romano, M., Luciotti, G., Gangemi, S., Marinucci, F., Prontera, C., D'Urbano, E. & Davi, G. (2002) Lab. Invest. 82, 1253–1254. [DOI] [PubMed] [Google Scholar]
- 24.Pirola, R., Bareggi, S. R. & De Benedittis, G. (1998) J. Chromatogr. B Biomed. Sci. Appl. 705, 309–315. [DOI] [PubMed] [Google Scholar]
- 25.Paulson, S. K., Hribar, J. D., Liu, N. W. K., Hajdu, E., Jr., Bible, R. H., Piergies, A. & Karim, A. (2000) Drug Metab. Dispos. 28, 308–314. [PubMed] [Google Scholar]
- 26.Conover, W. J. & Iman, R. L. (1981) Am. Stat. 35, 124–129. [Google Scholar]
- 27.Edgington, E. S. (1969) Statistical Inference: The Distribution-Free Approach (McGraw–Hill, New York), pp. 191–201.
- 28.Souza, M. H., de Lima, O. M., Jr., Zamuner, S. R., Fiorucci, S. & Wallace, J. L. (2003) Am. J. Physiol. 285, G54–G61. [DOI] [PubMed] [Google Scholar]