Abstract
Osteoblastic bone metastases are common in prostate and breast cancer patients, but mechanisms by which tumor cells stimulate new bone formation are unclear. We identified three breast cancer cell lines that cause osteoblastic metastases in a mouse model and secrete endothelin-1. Tumor-produced endothelin-1 stimulates new bone formation in vitro and osteoblastic metastases in vivo via the endothelin A receptor. Treatment with an orally active endothelin A receptor antagonist dramatically decreased bone metastases and tumor burden in mice inoculated with ZR-75-1 cells. Tumor-produced endothelin-1 may have a major role in the establishment of osteoblastic bone metastases, and endothelin A receptor blockade represents effective treatment.
Osteoblastic metastases occur in advanced cases of prostate cancer and frequently in breast cancer (1). Many factors have been proposed to cause disorganized new bone formation at sites of metastases, including insulin-like growth factors 1 and 2, transforming growth factor (TGF)-β, prostate-specific antigen, urokinase-type plasminogen activator, fibroblast growth factors (FGF)-1 and -2, bone morphogenic proteins (BMPs), and, in particular, endothelin-1 (ET-1) (2–7).
ET-1 is a potent vasoconstrictor that binds to ETA and ETB receptors with the latter functioning in ligand clearance (8, 9). ET-1 is produced by and affects bone cells (10–12). It stimulates mitogenesis in osteoblasts, which express both ETA and ETB receptors (13–15). ET-1 can decrease osteoclast activity and motility (16).
The prostate expresses ET-1 ligand and receptors (5–7). Primary and metastatic prostate cancers make ET-1 (5, 6, 17, 18), which can stimulate autocrine proliferation and potentiate effects of insulin-like growth factors, platelet-derived growth factor, epidermal growth factor, and FGF-2 (5). ETB receptor expression is decreased in prostate cancer (5).
Nelson et al. (6) found that plasma ET-1 concentrations were higher in men with advanced prostate cancer with bone metastases compared with men with organ-confined disease (6). ET-1 concentrations did not correlate to tumor burden in bone. Five human prostate cancer cell lines expressed ET-1 messenger RNA, and ET-1 increased BMP-initiated bone formation (6).
Breast cancers also express ET-1 and are the next most common cause of osteoblastic metastases. Breast cancer cells can convert preproET-1 to ET-1 (19, 20). Thus, substantial data implicate ET-1 in the pathogenesis of osteoblastic metastases, but a causal role for ET-1 in bone metastasis has not been directly tested. Questions remain about the importance of ET-1 on bone formation in vivo and whether ET-1 receptor blockade would decrease osteoblastic metastases.
We found three human breast cancer cell lines that produce ET-1 and cause osteoblastic bone metastases. We used nude mice inoculated with ZR-75-1 cells to demonstrate a causal role for ET-1 in osteoblastic metastasis. Endothelin A receptor blockade in this model dramatically decreased metastases and tumor burden in bone.
Materials and Methods
Cells. ZR-75-1 and T47D were from American Type Culture Collection. C. Kent Osborne (San Antonio, TX) provided MCF-7 and MDA-MB-231. ZR-75-1 and T47D cells were grown in RPMI medium 1640; MDA-MB-231 in DMEM; MCF-7 in Iscove's modified Eagle's medium (IMEM); and BT483, BT549, MDA-MB-435s, HS578T, MDA-MB-436, MDA-MB-361 PC-3, DU145, LNCaP, and TSU-Pr1 in 1:1 mixture of F12/DMEM. All media contained 10% FCS, 1% penicillin/streptomycin, and 1% nonessential amino acids in a 37°C atmosphere of 5% CO2/95% air. T47D and MCF-7 culture media were supplemented with insulin. At 80% confluence, 250 μl of serum-free media was conditioned in 48-well plates for 48 h, and cells were counted. ET-1 and BQ-123 were from American Peptide (Sunnyvale, CA).
New Bone Formation Assay in Murine Calvaria (21). Calvariae were excised from 4-day-old Swiss White mice, cut in half, and incubated in 1 ml of BGJ medium (Sigma) with 1% BSA containing respective factors or conditioned medium for 4 days (21). Bones were fixed, decalcified, paraffin-embedded, and sectioned. New bone area and osteoblast number were quantified on stained sections as described (21). ETA antagonist BQ-123, ETA antagonist ABT-627, ETB antagonist A-192621, and ETA/B antagonist A-182086 (Abbott Laboratories, Abbott Park, IL) were added to calvarial cultures 1 h before ET-1 or conditioned media. BGJ (25 ml) containing 1% BSA was conditioned for 48 h by near-confluent cells in T150 flasks; 20% media showed optimal effects on new bone formation and osteoblast proliferation.
Animals. Protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Female nude mice 4–6 weeks of age were housed in laminar flow isolated hoods.
Characterization of Osteoblastic Metastases. Mice were inoculated via the left cardiac ventricle (22) on day 0 with tumor cells that had been trypsinized, washed twice, and resuspended in PBS at 105 cells per 100 μl. Mice inoculated with PBS were controls. Radiographs were taken at the time of cell inoculation and every 3–4 weeks thereafter. At death (6 months after tumor inoculation for ZR-75-1 and 4 weeks for MDA-MB-231), bones and soft tissues were fixed in 10% buffered formalin for histology. Necropsy was performed. Mice with chest tumors (<10%) were not analyzed, as this indicated that the tumor cells did not enter the cardiac ventricle.
Radiography. Animals were x-rayed against the detector, and exposed with an x-ray at 35 KVP for 5 s by using a Faxitron Digital Radiographic Inspection unit (22).
Bone Histology and Histomorphometry. Fore- and hindlimb bones were fixed in 10% buffered formalin, decalcified in 10% EDTA, and embedded in paraffin. Sections were stained with hematoxylin, eosin, orange G, and phloxin. The following variables were measured in bone metastases: total tumor area, total bone area, new bone area, and osteoclast number per mm of tumor bone interface.
Effects of ABT-627 on Osteoblastic Bone Metastases. Mice were divided into six groups (n = 5 per group). Groups consisted of control PBS (groups a and d), ABT-627 2 mg/kg per day (groups b and e), and ABT-627 20 mg/kg per day (groups c and f). Groups a–c received MDA-MB-231 cells, and groups d–f received ZR-75-1 cells. ABT-627 was added to drinking water. Radiography, bone and soft tissue harvest, and autopsy were as in the previous experiment.
Mammary Fat Pad Assay. Cell suspensions (107 cells per 100 μl of PBS) of either ZR-75-1 or MDA-MB-231 cells were inoculated into the mammary fat pad of nude mice. Tumor volume was measured with calipers and calculated from the formula: tumor volume = 4/3 π × L/2 (W/2)2 (22).
Assays. ET-1 concentrations in conditioned media and plasma were determined by immunoassay (R & D Systems). ABT-627 (23) and parathyroid hormone-related protein (PTHrP) were measured as described (22). TGFβ-1 and -2 were measured in conditioned media by immunoassay (Promega). TGFβ bioactivity was measured by using mink lung epithelial cells transfected with a truncated PAI-1 promoter fused to luciferase (24).
Statistics. Results are expressed as mean ± SEM. Data were analyzed by repeated measures analysis of variance followed by Tukey–Kramer post hoc test. P < 0.05 was considered significant.
Results
ZR-75-1 Tumor Cells Cause Osteoblastic Metastases. Mice inoculated with ZR-75-1 cells developed radiographic-evident osteoblastic lesions over a period of 3–6 months (Fig. 1A). Histological sections from ZR-75-1 mice were compared with MDA-MB-231 and PBS controls (Fig. 1 B and C). ZR-75-1 mice had abundant new bone adjacent to metastatic tumor cells, whereas MDA-MB-231 had massive bone destruction. In both groups, metastases occurred in tibia, femur, spine, pelvis, scapula, and humerus. Histomorphometric analysis (Fig. 1 D–I) shows that total bone area in the ZR-75-1 group was 2-fold greater than PBS control. Total bone area was 3-fold greater compared with MDA-MB-231 (10.11 ± 0.78 vs. 5.69 ± 0.21 and 3.45 ± 0.27 mm2 per long bone, respectively; P < 0.01). Tumor area did not differ between ZR-75-1 and MDA-MB-231 groups. Tartrate-resistant acid phosphatase (TRAP)-stained sections revealed significantly fewer osteoclasts per mm of bone surface in the ZR-75-1 mice compared with PBS and MDA-MB-231 controls (0.32 ± 0.1 vs. 1.05 ± 0.08 and 3.28 ± 0.30 per mm of bone surface; P < 0.05). Osteoclast number per long bone was not significantly different between the PBS and ZR-75-1 groups. Thus, the increased bone associated with ZR-75-1 was due to more bone formation rather than less resorption (Fig. 1I).
Fig. 1.
Phenotype of bone metastases. (A) Radiographs of long bone and spine. (Upper) Hind limbs from mice inoculated with PBS (Left), MDA-MB-231 (Center), and ZR-75-1 (Right). (Lower) Spines. No lesions are evident in the PBS group (Left). Arrows designate osteolytic lesions in the MDA-MB-231 group (Center) and osteoblastic lesions in the ZR-75-1 group (Right). (B) Histology of distal femur from mice given PBS (Left), MDA-MB-231 (Center), and ZR-75-1 (Right). Tumor has replaced normal bone marrow and destroyed most of the trabecular and cortical bone in the MDA-MB-231 group. The marrow cavity is completely filled with new trabecular bone in the ZR-75-1 group. (C) Histology of vertebral bodies. Trabecular bone has replaced the marrow cavity in the ZR-75-1 group, whereas it has been destroyed in MDA-MB-231-bearing bone. (D–I) Histomorphometric analysis of bone area. (D) Bone area (mm2). ***, P < 0.0001 vs. PBS. (E) Tumor area (mm2). (F) Osteoclast number per mm of tumor bone interface. ***, P < 0.001 vs. PBS; #, P < 0.001 vs. MDA-MB-231. (G) Osteoclast number per mm of bone surface. ***, P < 0.001 vs. PBS; ****, P < 0.0001 vs. ZR-75-1; and P < 0.001 vs. PBS. (H) Total bone surface (mm). ***, P < 0.0001 vs. PBS. (I) Total osteoclast number per bone. ***, P < 0.001 vs. ZR-75-1 and PBS. D–F are hematoxylin- and eosin-stained sections, whereas G–I are sections stained with tartrate-resistant acid phosphatase. n = 10 per group.
ZR-75-1 conditioned medium stimulated new bone formation and osteoblast proliferation in mouse calvarial organ cultures, equivalently to BMP-2 positive control, whereas MDA-MB-231 conditioned medium had no effect on bone formation (Fig. 2A). Taken together, these results suggested that ZR-75-1 produces a factor that stimulates new bone formation and osteoblast proliferation.
Fig. 2.
ET-1 and ZR-75-1 stimulate new bone formation and osteoblast proliferation in organ cultures. (A) New bone area (Left) and osteoblast number (Right) from calvariae treated with conditioned media from ZR-75-1 and MDA-MB-231. *, P < 0.05 vs. control. (B) (Upper) Effect of BQ-123 (ETA antagonist) on ET-1-stimulated new bone formation (Left; **, P < 0.01 vs. control; ##, P < 0.01 vs. ET-1) and osteoblast number (Right; **, P < 0.01; *, P < 0.05 vs. control; #, P < 0.05 vs. ET-1). (Lower) Effect of BQ-123 (ETA antagonist) on ZR-75-1 stimulated new bone formation (Left; *, P < 0.05 vs. control; ##, P < 0.01 vs. ZR-75-1) and osteoblast number (Right; **, P < 0.01 vs. control; #, P < 0.05 vs. ZR-75-1). n = 4 per group. (C) Histology of calvariae: ET-1 (Left), ET-1 + BQ-123 (Center Left), ZR-75-1 (Center Right), ZR-75-1 + BQ-123 (Right). Note the increased new bone (arrows) and osteoblasts in ET-1- and ZR-75-1-treated bones. This effect was blocked by BQ-123.
Production of Bone-Stimulatory Factors by ZR-75-1 Cells. We assayed conditioned medium or RNA from ZR-75-1 and MDA-MB–231 cells for TGFβ-1 and -2, BMP-2, -3, -4, and -6, insulin-like growth factors 1 and 2, prostate-specific antigen, urokinase-type plasminogen activator, FGF-2, PTHrP, and ET-1. ET-1 was the only factor produced in excess by ZR-75-1 compared with MDA-MB-231 (Table 1).
Table 1. Bone-active factors produced by ZR-75-1 and MDA-MB-231 cell lines in vitro.
| ZR-75-1 | MDA-MB-231 | |
|---|---|---|
| Bone metastases | Osteoblastic | Osteolytic |
| TGFβ (latent) bioassay of CM | 0.43 ng per 105 cells | 0.96 ng per 105 cells per 48 h |
| TGFβ (active) bioassay of CM | - | - |
| TGFβ1 (latent) ELISA of CM | 0.42 ng per 105 cells | 0.71 ng per 105 cells per 48 h |
| TGFβ2 (latent) ELISA of CM | 0.13 ng per 105 cells | 0.65 ng per 105 cells per 48 h |
| BMP-2 (RNA) | - | 4+ |
| BMP-3 (RNA) | - | 4+ |
| BMP-4 (RNA) | 1+ | 4+ |
| BMP-6 (RNA) | - | 1+ |
| PTHrP IRMA of CM | - | 0.5-3 pM per 105 cells per 48 h |
| IGF-1 (RNA) | - | - |
| IGF-2 (RNA) | - | - |
| PSA (RNA) | - | - |
| PSA ELISA of CM | - | - |
| UPA | - | - |
| FGF-2 (RNA) | 2+ | - |
| FGF-2 (ELISA of CM) | - | - |
| ET-1 ELISA of CM | 80.7 ± 5.2 pg/ml per 105 cells per 48 h | - |
CM, conditioned media; IRMA, immunoradiometric assay; -, below detection limit; UPA, urokinase-type plasmogen activator; PSA, prostate-specific antigen.
ET-1 Is Responsible for New Bone Formation Induced by ZR-75-1. ZR-75-1 caused osteoblastic metastases in vivo, stimulated new bone formation and osteoblast proliferation in vitro, and produced ET-1. To determine whether ET-1 caused the new bone formation, we tested the effect of endothelin receptor blockade on ZR-75-1-stimulated new bone formation. An ETA receptor antagonist, BQ-123, blocked ET-1-stimulated new bone formation and osteoblast proliferation. BQ-123 had exactly the same effect in neonatal mouse calvarial cultures treated with ZR-75-1 conditioned media (Fig. 2B). These data indicate that ET-1 produced by ZR-75-1 is responsible for new bone formation and osteoblast proliferation.
Human Breast Cancer Cell Lines That Secrete ET-1 Cause Osteoblastic Metastases. Three additional breast cancer cell lines, T47D, MCF-7, and BT483, produced ET-1 (Table 2). MCF-7 and T47D caused osteoblastic metastases in 3 and 6 months, respectively (Fig. 3A), whereas BT483 caused sporadic mixed osteolytic and osteoblastic lesions. Conditioned media from MCF-7 and T47D stimulated new bone formation and osteoblast proliferation in neonatal mouse calvariae. The effect was again blocked by an ETA antagonist (Fig. 3B). Of the prostate cancer lines, only DU145 produced ET-1. None of the osteoblastic lines produced PTHrP, a mediator of osteolytic bone metastases (Table 2). All cell lines that caused osteolytic bone metastases secreted PTHrP. The results suggest that ET-1 may be a common cause of osteoblastic metastases.
Table 2. ET-1 production by cancer lines that cause bone metastases.
| Cell line | Bone metastases | ET-1, pg/ml per 105 cells per 48 h | [PTHrP], pM per 105 cells per 48 h |
|---|---|---|---|
| Breast cancers | |||
| ZR-75-1 | OB | 80.7 ± 5.2 | 0 |
| MCF-7 | OB | 18.9 ± 1.6 | 0 |
| T47D | OB | 227 ± 12.1 | 0 |
| BT483 | M | 22.3 ± 5.8 | 0 |
| MDA-MB-231 | OL | 0 | 0.54 ± 0.1 |
| BT549 | OL | 0 | 0.44 ± 0.12 |
| MDA-MB-435s | OL | 0 | 0.29 ± 0.12 |
| HS578T | N | 2.8 ± 2.1 | 0.46 ± 0.05 |
| MDA-MB-436 | N | 0 | 0.4 ± 0.4 |
| MDA-MB-361 | N | 0 | 0 |
| Prostate cancers | |||
| DU145 | N | 23.4 ± 1.5 | 0.75 ± 0.08 |
| LNCaP | N | 0 | 0.08 ± 0.08 |
| PC-3 | OL | 0 | 11.6 ± 0.82 |
OB, osteoblastic (with increased osteoid at tumor—bone interface); OL, osteolytic (with increased osteoclast numbers at tumor—bone interface); M, mixed (with regions of OB and OL in the same metastasis); N, none.
Fig. 3.
Osteoblastic bone metastases from MCF-7 and T47D are mediated by ET-1. (A) Histology of bones affected with osteoblastic metastases from MCF-7 and T47D. Tumor cells and new bone partially fill the marrow space in the sections from T47D-bearing mice (Right), whereas tumor and new bone have completely replaced the vertebral body marrow space in mice inoculated with MCF-7 (Center). (B) (Upper) Effects of T47D conditioned media on new bone area (Left) and osteoblast number (Right) in organ cultures. *, P < 0.05; **, P < 0.01 vs. control; and #, P < 0.05 vs. T47D. (Lower) Effects of MCF-7 conditioned media on new bone area (Left) and osteoblast number (Right). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. control; and #, P < 0.05 vs. MCF-7. ETA antagonist BQ-123 inhibited effects by both tumor cell lines.
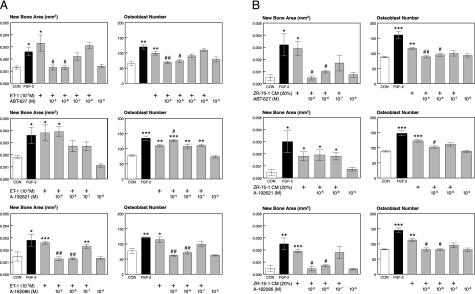
ET-1 Stimulates New Bone Formation via the ETA Receptor. We tested three ET receptor antagonists (26–28) on ET-1 stimulated new bone formation. ETA selective (ABT-627) and ETA/B nonselective (A-182086) compounds blocked new bone formation and osteoblast proliferation in a dose-dependent manner, in response to conditioned medium from ZR-75-1 cells or to ET-1. The ETB-selective antagonist A-192621 did not. ET-1 plus A-192621 stimulated osteoblast proliferation to a greater extent than ET-1 alone (Fig. 4 A and B). The blockade of ET-1-stimulated osteoblast proliferation and new bone formation by ABT-627 was specific, because it did not block equivalent responses to FGF-2 (data not shown). The experiments show that ET-1 can cause osteoblastic metastases, and its effects are mediated by the ETA receptor.
Fig. 4.
Role of ET receptors on new bone formation and osteoblast proliferation in organ culture. (A) Effects of ET receptor antagonists on ET-1-stimulated new bone formation. (Top) ABT-627, a selective ETA receptor antagonist, blocks ET-1-induced new bone formation. New bone area (Left) and osteoblast number (Right) are shown. *, P < 0.05; **, P < 0.01 vs. control; #, P < 0.05; and ##, P < 0.01 vs. ET-1. (Middle) A-192621, a selective ETB antagonist, has no effect on ET-1-stimulated new bone formation. New bone area (Left) and osteoblast number (Right) are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. control; and #, P < 0.05 vs. ET-1. (Bottom) Nonselective ETA/B receptor antagonist A-182086 blocked ET-1-stimulated new bone formation. New bone area (Left) and osteoblast number (Right) are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. control; and ##, P < 0.01 vs. ET-1. (B) Effects of antagonists on ZR-75-1-stimulated new bone formation. (Top)ETA antagonist ABT-627 blocked ZR-75-1-stimulated new bone formation. New bone area (Left; *, P < 0.05 vs. control; #, P < 0.05 vs. ZR-75-1) and osteoblast number (right; **, P < 0.01; ***, P < 0.001 vs. control; #, P < 0.05; and ##, P < 0.01 vs. ZR-75-1) are shown. (Middle)ETB antagonist A-192621 had no effect on ZR-75-1-stimulated new bone formation. New bone area (Left; *, P < 0.05 vs. control) and osteoblast number (Right; ***, P < 0.001 vs. control; #, P < 0.05 vs. ZR-75-1) are shown. (Bottom) Nonselective ETA/B receptor antagonist A-182086 blocked ZR-75-1-stimulated new bone formation. New bone area (Left; **, P < 0.01; ***, P < 0.001 vs. control; and #, P < 0.05 vs. ZR-75-1) and osteoblast number (Right; **, P < 0.01; ***, P < 0.001 vs. control; and #, P < 0.05 vs. ZR-75-1) are shown. n = 4 per group.
Effects of ETA Antagonist on Osteoblastic Metastases. We then tested the role of ET-1 in the development and progression of osteoblastic metastases in vivo. Nude mice were inoculated with ZR-75-1 cells and treated with ETA antagonist ABT-627 at two doses, 2 mg/kg per day and 20 mg/kg per day, or vehicle control. MDA-MB-231-inoculated mice were used as negative controls and treated similarly. ABT-627, a stable, orally active ETA receptor antagonist (25–30) was administered in drinking water. All mice in the control group had radiographic evidence of osteoblastic metastases by 26 weeks. No lesions were detectable in mice receiving ABT-627. Analysis of long bone, spine, and scapula revealed that both total bone and new bone area were significantly less in the treatment groups compared with control (P < 0.05) (Fig. 5 A–C). ABT-627 had no effect on osteolytic metastases caused by MDA-MB-231 (Fig. 5D) and caused no adverse effects in the animals. At time of death, serum concentrations of ABT-627 were undetectable in the vehicle control group, were 4.41 ± 0.49 ng/ml in the 2 mg/kg per day group, and were 45.5 ± 11.60 ng/ml in the 20 mg/kg per day group (P < 0.01).
Fig. 5.
Effect of ETA receptor anatagonist ABT-627 on osteoblastic metastases in vivo. (A) Six-month radiographs of mice inoculated with ZR-75-1. Treatment groups included vehicle control, ABT-627 2 mg/kg per day, and ABT-627 20 mg/kg per day administered in the drinking water. (Left) Osteoblastic lesion in the femur of a vehicle-treated mouse. We noted no lesions in ABT-627-treated mice (Center and Right). (B) Histology from femurs of A with new bone formation and tumor in the femur of the vehicle-treated mouse and no tumor or new bone in ABT-627-treated mice. (C) Bone histomorphometric analysis from mice in A. (Left) Total bone of long bone and spine was less in ABT-627-treated mice. P < 0.05. (Center) New bone area was greater in control mice vs. ABT-627-treated mice. P < 0.01. n = 5 per group. (Right) Tumor area in bone was significantly greater in control mice vs. ABT-627-treated mice. P < 0.05. (D) Bone histomorphometry from mice inoculated with MDA-MB-231 treated with ABT-627 for 4 weeks starting at tumor inoculation. We noted no difference in tumor area (Left) or total bone area (Right) between control and ABT-627-treated mice. (E) The effect of ABT-627 on ZR-75-1 or MDA-MB-231 mammary fat pad growth. Tumor cells were inoculated on day 0. n = 10 per group. No growth differences were noted.
Mice were inoculated in mammary fat pads with ZR-75-1 or MDA-MB-231 and treated with ABT-627 or vehicle. There was no effect of ABT-627 on growth of either ZR-75-1 or MDA-MB-231 (Fig. 5E). ZR-75-1 cells did not express ET receptor mRNAs by RT-PCR (data not shown). Thus the antimetastatic effect of ETA receptor blockade was not by direct inhibition of tumor cell growth.
Discussion
Bone is the most common site of cancer metastasis. Breast and prostate cancer account for >80% of cases of metastatic bone disease (1). In prostate cancer, the metastases are predominantly osteoblastic and cause pain, fracture, and nerve compression syndromes. Such lesions also occur in breast cancer. Previously, there have been no reproducible animal models of osteoblastic metastases, so the pathogenesis of these common bone lesions has been unclear. Our model causes consistent osteoblastic metastases in 100% of nude mice after inoculation of ZR-75-1 tumor cells. The model provides the opportunity to study the pathogenesis of tumor cell–bone interactions and the new bone formation characteristic of prostate and breast cancer metastases.
ZR-75-1 cells express ET-1. Both conditioned media and synthetic ET-1 peptide potently stimulated osteoblast proliferation and new bone formation in vitro. These responses were blocked by ETA (but not ETB) receptor antagonists. Treatment with orally active ABT-627, a selective ETA receptor antagonist, significantly reduced osteoblastic bone metastases and tumor burden in bone. ABT-627 had no effect on tumor growth in the mammary fat pad and was entirely without effect on ET-1 nonexpressing MDA-MB-231. Administration of ABT-627 in the drinking water gave serum concentrations of drug similar to levels achieved in patients with cancer (31).
These results demonstrate that tumor-produced ET-1 causes osteoblastic bone metastases by stimulating osteoblast proliferation and new bone formation via ETA receptors. Thus, ETA receptor blockade may be effective for prevention and treatment of osteoblastic bone metastases.
ABT-627 does not inhibit osteoblastic metastases by direct effects on the tumor cell, because ZR-75-1 cells express neither ETA nor ETB receptor mRNAs. The data indicate that ABT-627 blocks the responses of osteoblasts to tumor-produced ET-1. A major consequence of this is a dramatic reduction of tumor burden in bone, without increased tumor metastases to other organs. Osteoblasts stimulated by ET-1 may produce growth factors, which stimulate tumor cell growth, initiating a vicious cycle of more new bone formation and consequent morbidity. Le Brun et al. (32) showed that IL-1 β, TNFα, and TGFβ, which are produced locally in bone, increased ET-1 expression by human prostate cancer. Similarly, Granchi et al. (33) showed that ET-1 gene expression and protein secretion were increased in PC-3 and DU145 prostate cancer by TGFβ, epidermal growth factor, and IL-1α. Finally, prostate cancer cell production of ET-1 is enhanced by contact with bone (34).
Our results support those of Nelson et al. (7), which show that overexpression of ET-1 in the WISH tumor line, isolated from human amnion, resulted in increased new bone formation (7). However, another stimulator of osteoblast proliferation, FGF-2, has been purified from the WISH tumor (35). Nonetheless, ET-1 clearly stimulates osteoblast activity and new bone formation, either alone or in combination with other osteoblast mitogens. Our data indicate an important role for ET-1 in the pathogenesis of osteoblastic bone metastases, but other tumor-produced factors may also contribute. Platelet-derived growth factor BB caused new bone formation induced by MCF-7 cells, overexpressing the neu oncogene (36).
Our data suggest that the effects of ET-1 on bone metastases are mediated through the osteoblast. ET-1 may also affect tumor growth, apoptosis, invasion, and angiogenesis (37, 38). ETA receptor blockade reduced tumor growth of colon cancer in vivo (39). ET-1 induced tumor proteinase activation and invasiveness of ovarian cancer cells (40). It can also protect cancer cells from paclitaxel-induced apoptosis (41). ET-1 stimulates vascular endothelial growth factor (VEGF) synthesis via osteoblast-like MC3T3-E1 (42). Future investigation is needed to define the role of ET-1 in these steps of the metastastic cascade.
The endothelin axis offers an important target for cancer therapy (28). ABT-627 suppressed biochemical markers of bone formation and bone resorption, as well as changes in the bone scan index, in a recent study of 288 asymptomatic patients with hormone-refractory prostate cancer (29). Our preclinical studies, presented here, provide a mechanistic explanation for the clinical results.
We propose the following mechanism by which ET-1 promotes osteoblastic bone metastases: metastatic cancer cells in the bone microenvironment secrete ET-1, which binds to the ETA receptor and stimulates osteoblast proliferation and new bone formation. The stimulation of osteoblast activity enriches the local microenvironment with growth factors, which in turn could increase tumor burden and ET-1 secretion. This hypothesis proposes that the net effect is a vicious cycle, which increases osteoblastic bone metastases. In vitro evidence supports a role for TGFβ, produced by the osteoblast in response to ET-1 (32, 33), in the vicious cycle. Identification of the molecular mechanisms responsible for osteoblastic bone metastases is crucial for the effective treatment and prevention of this devastating complication of cancer.
Our animal model presented here conclusively shows that ETA receptor blockade is an effective treatment for osteoblastic bone metastases. Tumor-produced ET-1 stimulates new bone formation in vitro and osteoblastic metastases in vivo, and these effects are mediated via ETA receptors. ETA receptor blockade significantly reduces osteoblastic bone metastases and tumor burden in bone. The growing list of potential clinical applications for ETA receptor blockade (30) should now include osteoblastic bone metastases. Blockade for the ETA receptor may be effective in prevention and treatment of osteoblastic bone metastases caused by breast and prostate cancer.
Acknowledgments
We thank Marie Harris, Yong Cui, Barry Grubbs, Dr. Douglas Yee, Dr. Scott Cramer, Dr. Sarah Dallas, Dr. Carmine Lanni, Rami Käkönen, Suzanne Schoenfelt, Dana O'Konski, and Robin Rothkopf. This work was supported by National Institutes of Health Grants CA69158 and CA40035, the San Antonio Cancer Institute, and Abbott Laboratories.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TGF; transforming growth factor; FGF, fibroblast growth factor; BMP, bone morphogenic protein; ET-1, endothelin-1; PTHrP, parathyroid hormone-related protein.
See commentary on page 10588.
References
- 1.Guise, T. A. & Mundy, G. R. (1998) Endocr. Rev. 19, 18–55. [DOI] [PubMed] [Google Scholar]
- 2.Achbarou, A., Kaiser, S., Tremblay, G., Ste-Marie, L. G., Brodt, P., Goltzman, D. & Rabbani, S. A. (1994) Cancer Res. 54, 2372–2377. [PubMed] [Google Scholar]
- 3.Thalmann, G. N., Anezinis, P. E., Chang, S. M., Zhau, H. E., Kim, E. E., Hopwood, V. L., Pathak, S., von Eschenbach, A. C. & Chung, L. W. (1994) Cancer Res. 54, 2577–2581. [PubMed] [Google Scholar]
- 4.Gingrich, J. R., Barrios, R. J., Morton, R. A., Boyce, B. F., DeMayo, F. J., Finegold, M. J., Angelopoulou, R., Rosen, J. M. & Greenberg, N. M. (1996) Cancer Res. 56, 4096–4102. [PubMed] [Google Scholar]
- 5.Nelson, J. B., Chan-Tack, K., Hedican, S. P., Magnuson, S. R., Opgenorth, T. J., Bova, G. S. & Simons, J. W. (1996) Cancer Res. 56, 663–668. [PubMed] [Google Scholar]
- 6.Nelson, J. B., Hedican, S. P., George, D. J., Reddi, A. H., Piantadosi, S., Eisenberger, M. A. & Simons, J. W. (1995) Nat. Med. 1, 944–949. [DOI] [PubMed] [Google Scholar]
- 7.Nelson, J. B., Nguyen, S. H., Wu-Wong, J. R., Opgenorth, T. J., Dixon, D. B., Chung, L. W. & Inoue, N. (1999) Urology 53, 1063–1069. [DOI] [PubMed] [Google Scholar]
- 8.Yanagisawa, M., Kurihara, H., Kimura, S., Tomobe, Y., Kobayashi, M., Mitsui, Y., Yazaki, Y., Goto, K. & Masaki, T. (1988) Nature 332, 411–415. [DOI] [PubMed] [Google Scholar]
- 9.Levin, E. R. (1995) N. Engl. J. Med. 333, 356–363. [DOI] [PubMed] [Google Scholar]
- 10.Kitten, A. M. & Andrews, C. J. (2001) J. Cell. Physiol. 187, 218–225. [DOI] [PubMed] [Google Scholar]
- 11.Lam, H. C., Lee, J. K. & Lai, K. H. (2000) Endocrine 12, 77–80. [DOI] [PubMed] [Google Scholar]
- 12.Masukawa, H., Miura, Y., Sato, I., Oiso, Y. & Suzuki, A. (2001) J. Cell. Biochem. 83, 47–55. [DOI] [PubMed] [Google Scholar]
- 13.Stern, P. H., Tatrai, A., Semler, D. E., Lee, S. K., Lakatos, P., Strieleman, P. J., Tarjan, G. & Sanders, J. L. (1995) J. Nutr. 125 (Suppl.), 2028S–2032S. [DOI] [PubMed] [Google Scholar]
- 14.Kitano, Y., Kruihara, H., Kurihara, Y., Maemura, K., Ryo, Y., Yazaki, Y. & Harii, K. (1998) J. Bone Miner. Res. 13, 237–244. [DOI] [PubMed] [Google Scholar]
- 15.Kasperk, C. H., Borcsok, I., Schairer, H. U., Schneider, U., Nawroth, P. P., Niethard, F. U. & Ziegler, R. (1997) Calcif. Tissue Int. 60, 368–374. [DOI] [PubMed] [Google Scholar]
- 16.Alam, A. S., Gallagher, A., Shankar, V., Ghatei, M. A., Datta, H. K., Huang, C. L., Moonga, B. S., Chambers, T. J., Bloom, S. R. & Zaidi, M. (1992) Endocrinology 130, 3617–3624. [DOI] [PubMed] [Google Scholar]
- 17.de Matteis, A., Guidi, A., Di Paolo, B., Franco, G. & Revoltella, R. P. (2001) Cancer 91, 1933–1939. [DOI] [PubMed] [Google Scholar]
- 18.Corey, E., Quinn, J. E., Bladou, F., Brown L. G., Roudier, M. P., Brown, J. M., Buhler, K. R. & Vessella, R. L. (2002) Prostate 52, 20–33. [DOI] [PubMed] [Google Scholar]
- 19.Patel, K. V. & Schrey, M. P. (1995) Br. J. Cancer 71, 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yorimitsu, K., Moroi, K., Inagaki, N., Saito, T., Masuda, Y., Masaki, T., Seino, S. & Kimura, S. (1995) Biochem. Biophys. Res. Commun. 208, 721–727. [DOI] [PubMed] [Google Scholar]
- 21.Mundy, G., Garrett, R., Harris, S., Chan, J., Chen, D., Rossini, G., Boyce, B., Zhao, M. & Gutierrez, G. (1999) Science 286, 1946–1949. [DOI] [PubMed] [Google Scholar]
- 22.Yin, J. J., Selander, K., Chirgwin, J. M., Dallas, M., Grubbs, B. G., Wieser, R., Massague, J., Mundy, G. R. & Guise, T. A. (1999) J. Clin. Invest. 103, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryan, P. D., Sapochak, L. B., Tames, M. M., Padley, R. J. & El-Shourbagy, T. A. (2001) Biomed. Chromatogr. 15, 525–533. [DOI] [PubMed] [Google Scholar]
- 24.Abe, M., Harpel, J. G., Metz, C. N., Nunes, I., Loskutoff, D. J. & Rifkin, D. B. (1994) Anal. Biochem. 216, 276–284. [DOI] [PubMed] [Google Scholar]
- 25.Opgenorth, T. J., Adler, A. L., Calzadilla, S. V., Chiou, W. J., Dayton, B. D., Dixon, D. B., Gehrke, L. J., Hernandez, L., Magnuson, S. R. & Marsh, K. C., et al. (1996) J. Pharmacol. Exp. Ther. 276, 473–481. [PubMed] [Google Scholar]
- 26.von Geldern, T. W., Tasker, A. S., Sorensen, B. K., Winn, M., Szczepankiewicz, B. G., Dixon, D. B., Chiou, W. J., Wang, L., Wessale, J. L. & Adler, A., et al. (1999) J. Med. Chem. 42, 3668–3678. [DOI] [PubMed] [Google Scholar]
- 27.Jae, H. S., Winn, M., Dixon, D. B., Marsh, K. C., Nguyen, B., Opgenorth, T. J. & von Geldern, T. W. (1997) J. Med. Chem. 40, 3217–3227. [DOI] [PubMed] [Google Scholar]
- 28.Nelson, J., Bagnato, A., Battistini, B. & Nisen, P. (2003) Nat. Rev. Cancer 3, 110–116. [DOI] [PubMed] [Google Scholar]
- 29.Nelson, J., Nabulsi, A., Vogelzang, N., Breul, J., Zonnenberg, B., Daliani, D., Schulman, C. & Carducci, M. (2003) J. Urol. 169, 1143–1149. [DOI] [PubMed] [Google Scholar]
- 30.Remuzzi, G., Perico, N. & Benigni, A. (2002) Nat. Rev. Drug Discovery 1, 986–1001. [DOI] [PubMed] [Google Scholar]
- 31.Carducci, M. A., Nelson, J. B., Bowling, M. K., Rogers, T., Eisenberger, M. A., Sinibaldi, V., Donehower R., Leahy, T. L., Carr, R. A. & Isaacson, J. D., et al. (2002) J. Clin. Oncol. 20, 2171–2180. [DOI] [PubMed] [Google Scholar]
- 32.Le Brun, G., Aubin, P., Soliman, H., Ropiquet, F., Villette, J. M., Berthon, P., Creminon, C., Cussenot, O. & Fiet, J. (1999) Cytokine 11, 157–162. [DOI] [PubMed] [Google Scholar]
- 33.Granchi, S., Brocchi, S., Bonaccorsi, L., Baldi, E., Vinci, M. C., Forti, G., Serio, M. & Maggi, M. (2001) Prostate 49, 267–277. [DOI] [PubMed] [Google Scholar]
- 34.Chiao, J. W., Moonga, B. S., Yang, Y. M., Kancherla, R., Mittelman, A., Wu-Wong, J. R. & Ahmed, T. (2000) Br. J. Cancer 83, 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izbicka, E., Dunstan, C., Esparza, J., Jacobs, C., Sabatini, M. & Mundy, G. R. (1996) Cancer Res. 56, 633–636. [PubMed] [Google Scholar]
- 36.Yi, B., Williams, P. J., Niewolna, M., Wang, Y. & Yoneda, T. (2002) Cancer Res. 62, 917–923. [PubMed] [Google Scholar]
- 37.Salani, D., Taraboletti, G., Rosano, L., Di Castro, V., Borsotti, P., Giavazzi, R. & Bagnato, A. (2000) Am. J. Pathol. 157, 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salani, D., Di Castro, V., Nicotra, M. R., Rosano, L., Tecce R., Venuti, A., Natali, P. G. & Bagnato, A. (2000) Am. J. Pathol. 157, 1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asham, E., Shankar, A., Loizidou, M., Fredericks, S., Miller, K., Boulos, P. B., Burnstock, G. & Taylor, I. (2001) Br. J. Cancer 85, 1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosano, L., Varmi, M., Salani, D., Di Castro, V., Spinella, F., Natali, P. G. & Bagnato, A. (2001) Cancer Res. 61, 8340–8346. [PubMed] [Google Scholar]
- 41.Del Bufalo, D., Di Castro, V., Biroccio, A., Varmi, M., Salani, D., Rosano, L., Trisciuoglio, D., Spinella, F. & Bagnato, A. (2002) Mol. Pharmacol. 61, 524–532. [DOI] [PubMed] [Google Scholar]
- 42.Kozawa, O., Kawamura, H., Hatakeyama, D., Matsuno, H. & Uematsu, T. (2000) Cell. Signalling 12, 375–380. [DOI] [PubMed] [Google Scholar]