Abstract
Apolipoprotein (apo) E4 increases the risk and accelerates the onset of Alzheimer's disease (AD). However, the underlying mechanisms remain to be determined. We previously found that apoE undergoes proteolytic cleavage in AD brains and in cultured neuronal cells, resulting in the accumulation of carboxyl-terminal-truncated fragments of apoE that are neurotoxic. Here we show that this fragmentation is caused by proteolysis of apoE by a chymotrypsin-like serine protease that cleaves apoE4 more efficiently than apoE3. Transgenic mice expressing the carboxyl-terminal-cleaved product, apoE4(Δ272–299), at high levels in the brain died at 2–4 months of age. The cortex and hippocampus of these mice displayed AD-like neurodegenerative alterations, including abnormally phosphorylated tau (p-tau) and Gallyas silver-positive neurons that contained cytosolic straight filaments with diameters of 15–20 nm, resembling preneurofibrillary tangles. Transgenic mice expressing lower levels of the truncated apoE4 survived longer but showed impaired learning and memory at 6–7 months of age. Thus, carboxyl-terminal-truncated fragments of apoE4, which occur in AD brains, are sufficient to elicit AD-like neurodegeneration and behavioral deficits in vivo. Inhibiting their formation might inhibit apoE4-associated neuronal deficits.
Human apolipoprotein (apo) E, a 34-kDa protein composed of 299 amino acids, occurs as three major isoforms, apoE2, apoE3, and apoE4 (1–4). ApoE4 is a major risk or susceptibility factor for Alzheimer's disease (AD) (5–7). The apoE4 allele, which is found in 40–65% of cases of sporadic and familial AD, increases the occurrence and lowers the age of onset of the disease (7, 8).
Biochemical, cell biological, and transgenic animal studies have suggested several potential mechanisms to explain apoE4's contribution to the pathogenesis of AD. These include the modulation of the deposition and clearance of amyloid β peptides and the formation of plaques (9–15), impairment of the antioxidative defense system (16), dysregulation of neuronal signaling pathways (17), disruption of cytoskeletal structure and function (18, 19), and altered phosphorylation of tau and the formation of neurofibrillary tangles (NFTs) (20–23). However, the mechanisms of these apoE4-mediated detrimental effects are still largely unknown, and it is not known which are the primary effects and which are subsequent or downstream effects.
The neuropathological hallmarks of AD include extracellular amyloid plaques and intracellular NFTs in the brain (24–27). The plaques consist primarily of amyloid β peptides (24–26). The NFTs are composed largely of the highly phosphorylated microtubule-associated protein tau (p-tau) (25) and, to a lesser extent, of phosphorylated neurofilaments (28, 29). Both amyloid plaques and NFTs contain apoE (5, 30, 31); however, the role of apoE in the pathogenesis of these two lesions is uncertain. Histopathological and behavioral analyses of transgenic mice expressing different human apoE isoforms in the brain have revealed clear evidence for a dominant adverse effect of apoE4 (32–34), but the underlying mechanism is unknown.
Recently, we demonstrated that apoE is subject to proteolytic cleavage, resulting in bioactive carboxyl-terminal-truncated fragments (22). These apoE fragments are generated inside cultured neurons and in AD brains and can interact with p-tau and phosphorylated neurofilaments of high molecular weight, resulting in large, filamentous intracellular inclusions in neuronal cells (22).
We hypothesized that generation of these toxic apoE fragments is one of the early events in the pathogenesis of AD. In this study, we evaluated the pathogenic potential of the apoE fragments in vivo. Our results demonstrate that the carboxyl-terminal-truncated fragments of apoE4 elicited AD-like neurodegeneration and behavioral deficits in transgenic mice. Because apoE is synthesized by neurons under diverse pathological conditions (35–47), intraneuronal proteolytic processing of apoE could trigger apoE4-related neuropathology and promote the development of AD.
Materials and Methods
ApoE and Antibodies. Recombinant human apoE3 and apoE4 and anti-carboxyl-terminal apoE (amino acids 272–299) were prepared as described (22). Polyclonal goat anti-human apoE was purchased from Calbiochem. The phosphorylation-dependent monoclonal tau antibodies AT8 (p-Ser-202) and AT270 (p-Th-181) were purchased from Endogen (Cambridge, MA).
Human and Mouse Brain Tissues. Brain tissues from 17 nondemented subjects (10 apoE3/3, mean age 72 ± 6 years; seven apoE4/3, 70 ± 5 years) and 19 AD patients (nine apoE3/3, 75 ± 7 years; 10 apoE4/3 and apoE4/4, 72 ± 6 years) were collected at the Alzheimer Disease Research Center of the University of California at San Diego, at 5–14 h after death, frozen immediately on dry ice, and stored at –80°C until used. The tissue from the midfrontal gyrus (1–2 g) was homogenized with a Polytron homogenizer as described (22). The brain lysates (150 μg of total proteins) were subjected to SDS/PAGE and analyzed by anti-apoE Western blotting.
Brain tissues from 2- to 7-month-old wild-type and hemizygous transgenic mice were collected after a 2-min transcardial perfusion with PBS. One hemibrain from each mouse was homogenized and analyzed for apoE and p-tau as described (22). The other hemibrain was fixed in 3% paraformaldehyde, sectioned, and stained with hematoxylin and eosin, Gallyas silver, anti-apoE, and anti-p-tau as described (22, 34).
Partial Purification of ApoE-Cleaving Enzyme (AECE). Mouse brain homogenates or Neuro-2a cell lysates were passed through a DEAE column, and the bound proteins were eluted with a NaCl gradient (50–500 mM). The enzymatic activity of each fraction was assayed by incubating column fractions with recombinant apoE4 and observing the fragmentation pattern by SDS/PAGE and anti-apoE Western blotting. The AECE activity was eluted from the column at ≈200–250 mM NaCl. Fractions with AECE activity were pooled and passed through a Sephacryl S-200 gel filtration column. The AECE activity eluted primarily in fractions corresponding to proteins with molecular masses of 35–45 kDa (≈90% of AECE activity appeared in this peak, although silver-stained gels revealed several protein bands).
Assays of AECE Activity. For Western blotting, 1 μg of apoE4 was incubated with 5 μl of partially purified AECE in PBS (pH 7.4) at 37°C for 2 h, separated by SDS/PAGE, blotted to a nitrocellulose membrane, and probed with anti-apoE (22). For a chromogenic assay, chromogenic peptide substrates (50–100 μM; Sigma or Roche Diagnostics) were incubated with 5 μl of partially purified AECE in PBS (pH 7.4) at 37°C for 2–6 h, and absorbance at 410 nm was measured with a plate reader. For AECE inhibition studies, various protease inhibitors (1–10 mM; Sigma or Roche Diagnostics, see Fig. 1) were incubated with 5 μl of partially purified AECE in PBS (pH 7.4) at 37°C for 1 h and then with 1 μg of apoE4 at 37°C for 4 h. After incubation, apoE proteolysis was determined by SDS/PAGE and anti-apoE Western blotting.
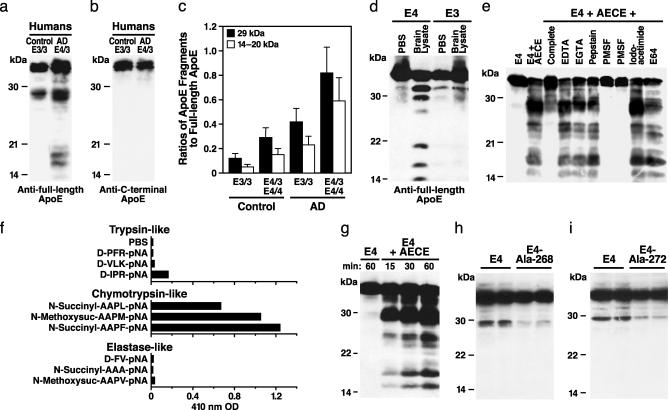
Fig. 1.
Cleavage of apoE by a chymotrypsin-like serine protease. ApoE fragmentation in human brain lysates was detected by Western blotting with antibodies against full-length apoE (a) or the carboxyl-terminal 28 amino acids of apoE (b) and quantified by densitometry (c). Brain tissues were from 17 nondemented subjects (10 apoE3/3, mean age 72 ± 6 years; seven apoE4/3, 70 ± 5 years) and 19 AD cases (nine apoE3/3, 75 ± 7 years; 10 apoE4/3 and apoE4/4, 72 ± 6 years). (d) ApoE fragmentation was assessed in vitro by incubating recombinant human apoE3 or apoE4 (1 μg) with brain lysate (20 μl) from apoE-deficient mice at 37°C for 3 h followed by anti-apoE Western blotting. (e) Effects of protease inhibitors (1–20 mM) on apoE4 cleavage by partially purified AECE. (f) Determination of substrate specificity of AECE with synthetic peptides (50–100 μM). (g) Time-dependent cleavage of apoE4 (1 μg) by partially purified AECE (5 μl) in vitro.(h) Effect of the Leu-268 to Ala mutation on apoE4 cleavage by AECE in transfected Neuro-2a cells. Neuro-2a cells were transiently transfected with cDNA constructs encoding wild-type apoE4 or apoE4–Ala-268. ApoE fragmentation was determined by SDS/PAGE and anti-apoE Western blotting 24 h after transfection. (i) Effect of the Met-272 to Ala mutation on apoE4 cleavage by AECE in Neuro-2a cells transfected with wild-type apoE4 or apoE4–Ala-272. ApoE fragmentation was determined by SDS/PAGE and anti-apoE Western blotting 24 h after transfection.
Generation of Truncated ApoE4 Constructs and Transgenic Mice. PCR products encoding human apoE4(Δ272–299) or apoE4(Δ241–299), both with a signal peptide for secretion and a FLAG tag at the amino terminus, were subcloned into a Thy-1.2 vector (kindly provided by H. van der Putten, Ciba-Geigy, Basel). Both DNA constructs were confirmed by DNA sequence analysis. Transgenic mice expressing apoE4(Δ272–299) or apoE4(Δ241–299) were generated by microinjecting these DNA constructs into C57BL/6J mouse eggs (Harlan Breeders, Indianapolis), as described (34). The presence and expression of transgenes were detected by PCR, in situ hybridization, and anti-apoE Western blotting (34). All hemizygous transgenic mice were on a pure C57BL/6J background and still expressed endogenous mouse apoE. Thus, wild-type mice were used as controls for all experiments.
Electron Microscopy. Mouse brain tissues were sectioned with a vibratome, fixed in 2.5% glutaraldehyde for 1 h and in 2% OsO4 for 1 h, dehydrated, embedded, sectioned, and stained with uranyl acetate and lead citrate. Cortical and hippocampal neurons were photographed with a JEOL JEM 100CX transmission electron microscope.
Behavioral Tests. Learning and memory were assessed with a Morris water maze test. Briefly, the ability of mice to locate a hidden platform submerged in a pool (122 cm in diameter) filled with opaque water (22°C) was determined in two sessions (2.5 h apart) per day for 5 days. Each session consisted of three consecutive trials. The platform location was constant for each mouse; the starting point at which the mouse was placed into the water was changed for each trial. On days 6–8, the ability of the mice to locate a visible platform was tested to exclude differences in vision, swim speed, and motivation. Decreases in the time it took the mice to reach the hidden platform (latencies) and decreasing path lengths were used as putative measures of spatial learning. On the mornings of days 3, 5, and 7, a probe trial (platform removed) was performed, and the time the mice spent in the quadrant where the platform was previously located was recorded as a measure of memory retention.
Emotionality was assessed with the elevated plus maze and basic motor function with rotorod tests as described (48).
Results
Isoform-Dependent Proteolysis of ApoE in Human Brains. To determine whether the fragmentation of apoE in AD brains is isoform-dependent, we measured the amounts of 29-kDa and 14- to 20-kDa fragments of apoE by anti-apoE Western blotting of brain lysates of AD cases that were homozygous for the most common apoE isoform, apoE3, or heterozygous or homozygous for apoE4 (Fig. 1 a–c). The ratios of 29-kDa and 14- to 20-kDa fragments to the full-length apoE (34-kDa) were higher in AD cases than in age- and sex-matched nondemented controls with the corresponding apoE genotypes (Fig. 1c, P < 0.01). Both AD and control cases with apoE4 had more apoE fragments than those without apoE4 (Fig. 1c, P < 0.01). This association between apoE fragmentation and AD raised several important questions.
ApoE4 Is More Susceptible than ApoE3 to Proteolysis in Vitro. We first examined whether apoE4 is also more susceptible to fragmentation than apoE3 in vitro. We incubated recombinant human apoE3 and apoE4 with lysates from apoE-deficient mouse brains (Fig. 1d) or from Neuro-2a cells (data not shown) and analyzed the fragmentation of apoE by anti-apoE Western blotting. ApoE-deficient mice were used in this experiment to avoid confusion of mouse and human apoEs. Polyclonal anti-apoE revealed full-length apoE and the 29-kDa and 14- to 20-kDa apoE fragments in apoE samples exposed to brain lysates (Fig. 1d). The fragments were detected by antibodies against the full-length apoE but not by an antibody against the carboxyl terminus of apoE (amino acids 272–299), indicating that they are carboxyl-terminal-truncated forms of apoE (data not shown). More fragments were generated from apoE4 than from apoE3 (Fig. 1d). These results raised the intriguing possibility that an AECE in mouse brain and Neuro-2a cells preferentially cleaves apoE4 to generate carboxyl-terminal-truncated fragments similar to those seen in human AD brains.
AECE Is a Chymotrypsin-Like Serine Protease. We partially purified the putative AECE from apoE-deficient mouse brains and from Neuro-2a cells and tested it with inhibitors of the four major categories of proteases. EDTA and EGTA did not inhibit the cleavage of apoE4 (Fig. 1e), suggesting that the enzyme is not a metalloprotease. Likewise, pepstatin and iodoacetamide or E-64 did not inhibit the cleavage (Fig. 1e), indicating that the enzyme is not an aspartate protease or a cysteine protease. However, PMSF abolished the cleavage activity, suggesting that the enzyme is a serine protease (Fig. 1e). A complete inhibitor mixture containing PMSF also significantly inhibited the cleavage of apoE4.
Serine proteases can be categorized into three groups: trypsin-like, chymotrypsin-like, and elastase-like. To test the specificity of the AECE, we incubated the partially purified AECE with chromogenic tri- or tetra-peptides containing arginine/lysine (Arg/Lys or R/K), phenylalanine/methionine/leucine (Phe/Met/Leu or F/M/L), or valine/alanine (Val/AlaorV/A) at the carboxyl-terminal end, which are substrates for trypsin-like, chymotrypsin-like, and elastase-like serine proteases, respectively (49). The partially purified AECE cleaved the carboxyl end of hydrophobic amino acids with an aromatic side chain (F) or with a larger side chain (L or M) (Fig. 1f), suggesting that the putative AECE is a chymotrypsin-like serine protease.
Leu-268 and Met-272 Are the Primary Cleavage Sites in ApoE4. When apoE4 was incubated with the partially purified AECE for various times, the 29-kDa-fragment was generated before the 14- to 20-kDa fragments (Fig. 1g), indicating that the 29-kDa fragment might be the first product of the proteolysis and the 14- to 20-kDa fragments might be derived from the 29-kDa fragment. None of the fragments reacted with the antibodies that recognized only the carboxyl-terminal 28 amino acids. The apparent molecular mass of the 29-kDa apoE fragments and the substrate specificity of AECE suggest that the primary cleavage site in apoE4 was probably at Phe-265, Leu-268, or Met-272. To test these possibilities, we produced a series of mutations. Mutating Leu-268 or Met-272 to Ala decreased the susceptibility of apoE4 to proteolysis by 78 ± 11% and 72 ± 9%, respectively, compared with wild-type apoE4 (Fig. 1 h and i), and mutating Phe-265 to Ala enhanced apoE4's susceptibility to cleavage (data not shown), suggesting that Leu-268 and Met-272 are the primary cleavage sites.
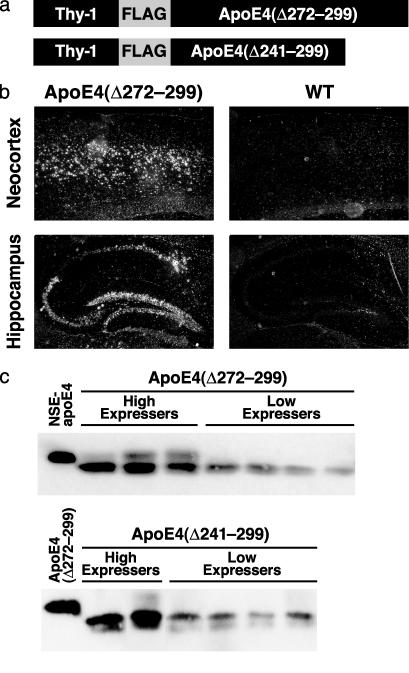
AD-Like Neurodegeneration in Transgenic Mice Expressing ApoE4(Δ272–299). The carboxyl-terminal-truncated apoE induces NFT-like inclusions in cultured neuronal cells (22, 23). To assess the pathogenic potential of the carboxyl-terminal-truncated apoE in vivo, we established transgenic mouse lines expressing apoE4(Δ272–299) at different levels in the brain, including levels similar to those in the brains of previously established neuron-specific enolase–apoE mice (Fig. 2c, high expressers) and to those in human cortex (34). To avoid the potential for neurotoxic effects of the truncated apoE4 (22) on embryonic development, we used a neuron-specific Thy-1 promoter that initiates transgene expression around day 15 after birth (Fig. 2a) (21). In situ hybridization demonstrated that the transgene was expressed in the cortex (Fig. 2b), hippocampus (Fig. 2b), cerebellum, olfactory bulb, and spinal cord (data not shown) in all transgenic founder mice (n = 9). All four founders expressing the truncated apoE4(Δ272–299) at high levels (Fig. 2c) died at 2–4 months of age. Anti-apoE immunostaining demonstrated expression of the carboxyl-terminal-truncated apoE4 in neurons in the neocortex (Fig. 3A), hippocampus (Fig. 3 B and C), cerebellum, and spinal cord (data not shown). These mice also had neuronal inclusion bodies containing truncated apoE4 (Fig. 3 A–C). Hematoxylin and eosin staining revealed degeneration of the neurons expressing the truncated apoE4 in the CA1 (Fig. 3D) and CA3 (Fig. 3E) subfields of the hippocampus.
Fig. 2.
Expression of human apoE4(Δ272–299) or apoE4(Δ241–299) in neurons of transgenic mice. (a) Thy-1-apoE cDNA constructs used for generating transgenic mice. (b) In situ hybridization of human apoE mRNA in the neocortex and hippocampus of transgenic mice expressing apoE4(Δ272–299) and nontransgenic wild-type (WT) controls. (c) Human apoE-specific Western blot analysis of brain homogenates from different founder mice. NSE, neuron-specific enolase.
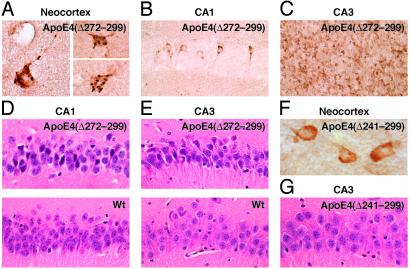
Fig. 3.
Neurodegeneration in the brains of hemizygous transgenic mice expressing apoE4(Δ272–299) in neurons. Brain sections from 2- to 4-month-old transgenic mice (C57BL/6J background) expressing high levels of apoE4(Δ272–299) (A–E) or apoE4(Δ241–299) (F and G) at comparable levels were stained with anti-apoE (A–C and F) or hematoxylin and eosin (D, E, and G). Nontransgenic wild-type (Wt) mice (D and E) served as additional controls. Note the formation of truncated apoE4-containing inclusions in cortical (A), CA1 (B), and CA3 (C) neurons and degeneration of neurons in CA1 (D Upper) and CA3 (E Upper) in apoE4(Δ272–299) mice. Original magnifications: A, C, and F, ×600; B, D, E, and G, ×400.
Importantly, mice with high-level expression of apoE4(Δ272–299) had neurodegeneration, whereas mice expressing a shorter, truncated apoE4(Δ241–299) lacking the carboxyl-terminal 59 amino acids at similar levels (Fig. 2c, high expressers) did not develop either intracellular inclusion bodies in neurons or neurodegeneration (Fig. 3 F and G). These results suggest that neurodegeneration is not caused simply by expressing a truncated form of apoE4, but specifically by expressing the truncated apoE4(Δ272–299) that contains the lipid-binding region (amino acids 244–272) (2). The importance of this region of apoE in neurotoxicity is consistent with our previous in vitro observations (22).
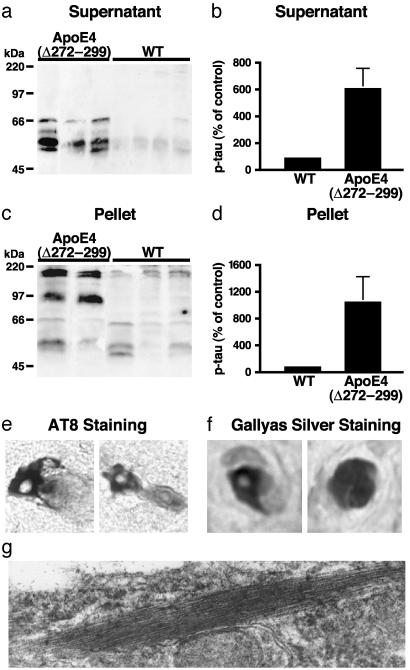
Monomeric p-tau and SDS-resistant p-tau polymers accumulated in the brains of the high-expresser apoE4(Δ272–299) mice at 2–4 months of age, as determined by Western blotting with anti-p-tau (Fig. 4 a and c). The p-tau levels were 6- to 11-fold higher in the high expressers than in nontransgenic littermates at the same age (Fig. 4 b and d). Thus, the truncated apoE4 elicits abnormal phosphorylation of tau in vivo. Neurons in the neocortex (Fig. 4e) and hippocampus (data not shown) were labeled with the AT8 antibody (Fig. 4e), which recognizes abnormally phosphorylated tau (21). They were also labeled by Gallyas silver staining (Fig. 4f) and contained cytosolic straight filaments with diameters of 15–20 nm (Fig. 4g), resembling pre-NFTs in AD brains (50). Nontransgenic mice of similar ages had no such alterations (data not shown).
Fig. 4.
Hyperphosphorylation of tau and formation of preNFT-like filaments in the brains of hemizygous transgenic mice expressing apoE4(Δ272–299). P-tau in the supernatants (a and b) and the solubilized pellets (c and d) of brain lysates of 2- to 4-month-old wild-type (n = 6) or high-expresser apoE4(Δ272–299) (n = 4) mice was detected by Western blotting with monoclonal antibody AT8 (a and c) and quantified by densitometry (b and d). (e) Cortical neurons positive for AT8 immunostaining in transgenic mice. (f) Cortical neurons positive for Gallyas silver staining in transgenic mice. (g) Intraneuronal straight filaments with diameters of 15–20 nm visualized by electron microscopy in transgenic mice. Original magnifications: e and f, ×600; g, ×60,000.
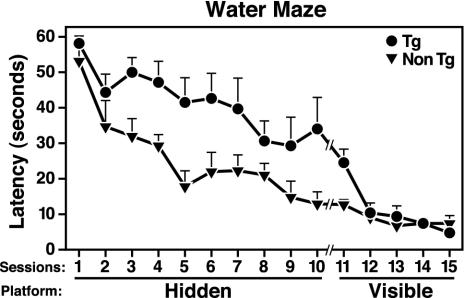
Transgenic Mice Expressing Lower Levels of ApoE4(Δ272–299) Show Deficits in Spatial Learning and Memory. Transgenic founder mice (n = 5) expressing lower levels of apoE4(Δ272–299) were viable and fertile. However, in a water maze test, they required longer times to reach the hidden target platform (escape latency) (Fig. 5), indicating impaired spatial learning and memory. Analysis of the path lengths of the mice confirmed the impairment of transgenic mice for the hidden (P < 0.05) but not the visible (P > 0.05) platform. There was no significant difference in the swimming speeds of transgenic and nontransgenic mice (data not shown). Furthermore, after 3 days of hidden platform training, the nontransgenic mice, but not the transgenic mice, showed a significant preference for the target quadrant during the probe trial, indicating impaired memory retention of the transgenic mice. Transgenic and nontransgenic mice showed comparable probe trial performance after 5 days of training. Transgenic and nontransgenic mice performed similarly in the plus maze test (63 ± 17 versus 69 ± 22 s) and rotorod test (74 ± 13 versus 82 ± 10 s), indicating that the impairments of transgenic mice in the water maze test were not caused by changes in emotionality or motor functions.
Fig. 5.
Learning and memory deficits of hemizygous transgenic mice expressing low levels of apoE4(Δ272–299). Five transgenic (Tg) founder mice (C57BL/6J) expressing low levels of apoE4(Δ272–299) on a wild-type mouse apoE background and five nontransgenic (Non Tg) wild-type littermates (all males) from the same founder generation were tested in the water maze at 6–7 months of age. Mice were tested in two sessions per day (three trials for each session). The y axis indicates the time to reach the target platform (mean ± SEM). For sessions 1–10, the platform was hidden under the opaque water in a constant location. For sessions 11–15, the platform was visible and changed in location between sessions. Repeated-measures ANOVA revealed significant differences in the learning curves of transgenic and nontransgenic mice for the hidden (P < 0.01), but not the visible (P > 0.05), component of the water maze test.
Discussion
This study demonstrates that apoE4 is more susceptible than apoE3 to proteolytic cleavage by a chymotrypsin-like serine protease and that the resulting bioactive carboxyl-terminal-truncated fragments induce AD-like neuropathology and behavioral deficits in transgenic mice. It is tempting to speculate that the neuronal alterations elicited by the expression of the truncated apoE4 in transgenic mice relate to neuronal alterations observed in AD and in transgenic mice expressing full-length apoE4 in neurons (32–34).
Importantly, apoE3 was also cleaved by the putative AECE, albeit less effectively than apoE4. Thus, AECE-generated apoE fragments might contribute to the development of neuronal deficits also in APOE ε3 carriers, although it would take longer for adverse effects to become apparent than in APOE ε4 carriers, consistent with the different effects of apoE3 and apoE4 on AD onset (7, 8). Our previous studies suggested that apoE fragments can exert adverse effects on the cytoskeleton (22). Alternatively, apoE fragments might contribute to AD pathogenesis more indirectly by increasing the susceptibility of neurons to copathogens. These possibilities are not mutually exclusive.
Neurodegeneration was not caused simply by expressing a truncated form of apoE4 but specifically by expressing apoE4(Δ272–299), which contains the lipid-binding region (amino acids 244–272) (2). The importance of this region of apoE in neurotoxicity is consistent with our previous in vitro observations in which the lipid-binding region of apoE is toxic to cultured neuronal cells (22). The amino-terminal 22-kDa thrombin-cleavage fragment (amino acids 1–191) of apoE4 is also neurotoxic in vitro (51, 52), but this toxicity appears to require relatively high concentrations of the fragment and has not yet been confirmed in vivo. The apoE fragments generated in AD brains are different from the 22-kDa thrombin-cleavage products (22), which lack the lipid-binding domain (amino acids 244–272). Indeed, the lipid-binding domain is present in all of the major apoE fragments generated in AD brains (22) and, in light of the current study, appears to be essential for apoE fragments to have neurotoxic effects in vivo. Interestingly, the lipid-binding domain of apoE is also responsible for interaction of apoE with amyloid β peptides (9, 53). Thus, it is reasonable to speculate that the apoE fragments released from neurons might also interact with amyloid β and act synergistically to induce neuronal deficits.
Initially, apoE was thought to be synthesized in the brain by astrocytes, oligodendrocytes, activated microglia, and ependymal layer cells, but not by neurons (54). However, numerous subsequent studies have demonstrated that CNS neurons can also express apoE, albeit at lower levels than astrocytes (35–47). Both apoE protein and mRNA are found in cortical and hippocampal neurons in humans (42) and in transgenic mice expressing human apoE under the control of the human apoE promoter (43). In rats treated with kainic acid, apoE expression is induced in hippocampal neurons that survive excitotoxic stress, as determined by both in situ hybridization and anti-apoE immunohistochemistry (44). Furthermore, expression of neuronal apoE can be induced in human brains after cerebral infarction (47). ApoE is also expressed in primary cultured human CNS neurons (45) and in many human neuronal cell lines, including SY-5Y, Kelly, and NT2 cells (46, 55–57). CNS injury may induce neuronal expression of apoE to participate in neuronal repair or remodeling or to protect neurons from injury (44). However, in neurons expressing apoE4, the proteolytic processing and fragment generation identified by our studies may turn a neuroprotective response into a pathogenic process.
In conclusion, this study demonstrates that the carboxyl-terminal-truncated fragments of apoE4 elicit AD-like neurodegeneration and behavioral deficits in transgenic mice. We hypothesize that apoE4 produced in neurons under diverse pathological conditions is cleaved by AECE. The resulting bioactive carboxyl-terminal-truncated fragments, probably together with other AD-related factors (e.g., amyloid β peptides), induce neuropathology and behavioral deficits characteristic of AD. Consequently, AECE represents a potential new target for drugs aimed at inhibiting the detrimental effects of apoE4 in AD (58) and other neurological diseases (59–61).
Acknowledgments
We thank Dr. Sharon Grehan for assisting with in situ hybridization experiments, Dr. Thomas Innerarity for critical reading of the manuscript, Jennifer Polizzotto and Sylvia Richmond for manuscript preparation, Stephen Ordway and Gary Howard for editorial assistance, John C. W. Carroll and John Hull for graphics, and Stephen Gonzales and Chris Goodfellow for photography. This work was supported in part by National Institutes of Health Program Project Grant P01 AG022074.
Abbreviations: AD, Alzheimer's disease; apo, apolipoprotein; AECE, apoE-cleaving enzyme; NFTs, neurofibrillary tangles; p-tau, phosphorylated tau.
References
- 1.Mahley, R. W. (1988) Science 240, 622–630. [DOI] [PubMed] [Google Scholar]
- 2.Mahley, R. W. & Huang, Y. (1999) Curr. Opin. Lipidol. 10, 207–217. [DOI] [PubMed] [Google Scholar]
- 3.Huang, Y. & Mahley, R. W. (1999) in Plasma Lipids and Their Role in Disease, eds. Barter, P. J. & Rye, K.-A. (Harwood Academic, Amsterdam), pp. 257–284.
- 4.Mahley, R. W. & Rall, S. C., Jr. (2000) Annu. Rev. Genomics Hum. Genet. 1, 507–537. [DOI] [PubMed] [Google Scholar]
- 5.Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S. & Roses, A. D. (1993) Proc. Natl. Acad. Sci. USA 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roses, A. D. (1994) J. Neuropathol. Exp. Neurol. 53, 429–437. [DOI] [PubMed] [Google Scholar]
- 7.Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., Roses, A. D., Haines, J. L. & Pericak-Vance, M. A. (1993) Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- 8.Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., Myers, R. H., Pericak-Vance, M. A., Risch, N. & Van Duijn, C. M. (1997) J. Am. Med. Assoc. 278, 1349–1356. [PubMed] [Google Scholar]
- 9.Strittmatter, W. J., Weisgraber, K. H., Huang, D. Y., Dong, L.-M., Salvesen, G. S., Pericak-Vance, M., Schmechel, D., Saunders, A. M., Goldgaber, D. & Roses, A. D. (1993) Proc. Natl. Acad. Sci. USA 90, 8098–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma, J., Yee, A., Brewer, H. B., Jr., Das, S. & Potter, H. (1994) Nature 372, 92–94. [DOI] [PubMed] [Google Scholar]
- 11.Wisniewski, T., Castaño, E. M., Golabek, A., Vogel, T. & Frangione, B. (1994) Am. J. Pathol. 145, 1030–1035. [PMC free article] [PubMed] [Google Scholar]
- 12.LaDu, M. J., Falduto, M. T., Manelli, A. M., Reardon, C. A., Getz, G. S. & Frail, D. E. (1994) J. Biol. Chem. 269, 23403–23406. [PubMed] [Google Scholar]
- 13.Holtzman, D. M., Bales, K. R., Tenkova, T., Fagan, A. M., Parsadanian, M., Sartorius, L. J., Mackey, B., Olney, J., McKeel, D., Wozniak, D. & Paul, S. M. (2000) Proc. Natl. Acad. Sci. USA 97, 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bales, K. R., Verina, T., Cummins, D. J., Du, Y., Dodel, R. C., Saura, J., Fishman, C. E., DeLong, C. A., Piccardo, P., Petegnief, V., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 15233–15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irizarry, M. C., Cheung, B. S., Rebeck, G. W., Paul, S. M., Bales, K. R. & Hyman, B. T. (2000) Acta. Neuropathol. 100, 451–458. [DOI] [PubMed] [Google Scholar]
- 16.Miyata, M. & Smith, J. D. (1996) Nat. Genet. 14, 55–61. [DOI] [PubMed] [Google Scholar]
- 17.Herz, J. & Beffert, U. (2000) Nat. Rev. Neurosci. 1, 51–58. [DOI] [PubMed] [Google Scholar]
- 18.Nathan, B. P., Bellosta, S., Sanan, D. A., Weisgraber, K. H., Mahley, R. W. & Pitas, R. E. (1994) Science 264, 850–852. [DOI] [PubMed] [Google Scholar]
- 19.Nathan, B. P., Chang, K.-C., Bellosta, S., Brisch, E., Ge, N., Mahley, R. W. & Pitas, R. E. (1995) J. Biol. Chem. 270, 19791–19799. [DOI] [PubMed] [Google Scholar]
- 20.Strittmatter, W. J., Saunders, A. M., Goedert, M., Weisgraber, K. H., Dong, L.-M., Jakes, R., Huang, D. Y., Pericak-Vance, M., Schmechel, D. & Roses, A. D. (1994) Proc. Natl. Acad. Sci. USA 91, 11183–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tesseur, I., Van Dorpe, J., Spittaels, K., Van den Haute, C., Moechars, D. & Van Leuven, F. (2000) Am. J. Pathol. 156, 951–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, Y., Liu, X. Q., Wyss-Coray, T., Brecht, W. J., Sanan, D. A. & Mahley, R. W. (2001) Proc. Natl. Acad. Sci. USA 98, 8838–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ljungberg, M. C., Dayanandan, R., Asuni, A., Rupniak, T. H., Anderton, B. H. & Lovestone, S. (2002) NeuroReport 13, 867–870. [DOI] [PubMed] [Google Scholar]
- 24.Selkoe, D. J. (1991) Neuron 6, 487–498. [DOI] [PubMed] [Google Scholar]
- 25.Crowther, R. A. (1993) Curr. Opin. Struct. Biol. 3, 202–206. [Google Scholar]
- 26.Roses, A. D. (1994) Curr. Neurol. 14, 111–141. [Google Scholar]
- 27.Tanzi, R. E. & Bertram, L. (2001) Neuron 32, 181–184. [DOI] [PubMed] [Google Scholar]
- 28.Smith, M. A., Rudnicka-Nawrot, M., Richey, P. L., Praprotnik, D., Mulvihill, P., Miller, C. A., Sayre, L. M. & Perry, G. (1995) J. Neurochem. 64, 2660–2666. [DOI] [PubMed] [Google Scholar]
- 29.Vickers, J. C., Riederer, B. M., Marugg, R. A., Buée-Scherrer, V., Buée, L., Delacourte, A. & Morrison, J. H. (1994) Neuroscience 62, 1–13. [DOI] [PubMed] [Google Scholar]
- 30.Namba, Y., Tomonaga, M., Kawasaki, H., Otomo, E. & Ikeda, K. (1991) Brain Res. 541, 163–166. [DOI] [PubMed] [Google Scholar]
- 31.Wisniewski, T. & Frangione, B. (1992) Neurosci. Lett. 135, 235–238. [DOI] [PubMed] [Google Scholar]
- 32.Raber, J., Wong, D., Buttini, M., Orth, M., Bellosta, S., Pitas, R. E., Mahley, R. W. & Mucke, L. (1998) Proc. Natl. Acad. Sci. USA 95, 10914–10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raber, J., Wong, D., Yu, G.-Q., Buttini, M., Mahley, R. W., Pitas, R. E. & Mucke, L. (2000) Nature 404, 352–354. [DOI] [PubMed] [Google Scholar]
- 34.Buttini, M., Orth, M., Bellosta, S., Akeefe, H., Pitas, R. E., Wyss-Coray, T., Mucke, L. & Mahley, R. W. (1999) J. Neurosci. 19, 4867–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beffert, U. & Poirier, J. (1996) Ann. N.Y. Acad. Sci. 777, 166–174. [DOI] [PubMed] [Google Scholar]
- 36.Beisiegel, U., Schneider, W. J., Goldstein, J. L., Anderson, R. G. W. & Brown, M. S. (1981) J. Biol. Chem. 256, 11923–11931. [PubMed] [Google Scholar]
- 37.Diedrich, J. F., Minnigan, H., Carp, R. I., Whitaker, J. N., Race, R., Frey, W., II, & Haase, A. T. (1991) J. Virol. 65, 4759–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han, S.-H., Einstein, G., Weisgraber, K. H., Strittmatter, W. J., Saunders, A. M., Pericak-Vance, M., Roses, A. D. & Schmechel, D. E. (1994) J. Neuropathol. Exp. Neurol. 53, 535–544. [DOI] [PubMed] [Google Scholar]
- 39.Bao, F., Arai, H., Matsushita, S., Higuchi, S. & Sasaki, H. (1996) NeuroReport 7, 1733–1739. [DOI] [PubMed] [Google Scholar]
- 40.Metzger, R. E., LaDu, M. J., Pan, J. B., Getz, G. S., Frail, D. E. & Falduto, M. T. (1996) J. Neuropathol. Exp. Neurol. 55, 372–380. [DOI] [PubMed] [Google Scholar]
- 41.Xu, P.-T., Schmechel, D., Qiu, H.-L., Herbstreith, M., Rothrock-Christian, T., Eyster, M., Roses, A. D. & Gilbert, J. R. (1999) Neurobiol. Dis. 6, 63–75. [DOI] [PubMed] [Google Scholar]
- 42.Xu, P.-T., Gilbert, J. R., Qiu, H.-L., Ervin, J., Rothrock-Christian, T. R., Hulette, C. & Schmechel, D. E. (1999) Am. J. Pathol. 154, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu, P.-T., Schmechel, D., Rothrock-Christian, T., Burkhart, D. S., Qiu, H.-L., Popko, B., Sullivan, P., Maeda, N., Saunders, A. M., Roses, A. D. & Gilbert, J. R. (1996) Neurobiol. Dis. 3, 229–245. [DOI] [PubMed] [Google Scholar]
- 44.Boschert, U., Merlo-Pich, E., Higgins, G., Roses, A. D. & Catsicas, S. (1999) Neurobiol. Dis. 6, 508–514. [DOI] [PubMed] [Google Scholar]
- 45.Dekroon, R. M. & Armati, P. J. (2001) Glia 33, 298–305. [DOI] [PubMed] [Google Scholar]
- 46.Dupont-Wallois, L., Soulié, C., Sergeant, N., Wavrant-de Wrieze, N., Chartier-Harlin, M.-C., Delacourte, A. & Caillet-Boudin, M.-L. (1997) Neurobiol. Dis. 4, 356–364. [DOI] [PubMed] [Google Scholar]
- 47.Aoki, K., Uchihara, T., Sanjo, N., Nakamura, A., Ikeda, K., Tsuchiya, K. & Wakayama, Y. (2003) Stroke 34, 875–880. [DOI] [PubMed] [Google Scholar]
- 48.Raber, J., Akana, S. F., Bhatnagar, S., Dallman, M. F., Wong, D. & Mucke, L. (2000) J. Neurosci. 20, 2064–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein, P. E. & Carrell, R. W. (1995) Struct. Biol. 2, 96–113. [DOI] [PubMed] [Google Scholar]
- 50.Hutton, M., Lewis, J., Dickson, D., Yen, S.-H. & McGowan, E. (2001) Trends Mol. Med. 7, 467–470. [DOI] [PubMed] [Google Scholar]
- 51.Tolar, M., Marques, M. A., Harmony, J. A. K. & Crutcher, K. A. (1997) J. Neurosci. 17, 5678–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tolar, M., Keller, J. N., Chan, S., Mattson, M. P., Marques, M. A. & Crutcher, K. A. (1999) J. Neurosci. 19, 7100–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho, H. S., Hyman, B. T., Greenberg, S. M. & Rebeck, G. W. (2001) J. Neuropathol. Exp. Neurol. 60, 342–349. [DOI] [PubMed] [Google Scholar]
- 54.Boyles, J. K., Pitas, R. E., Wilson, E., Mahley, R. W. & Taylor, J. M. (1985) J. Clin. Invest. 76, 1501–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartman, R. E., Wozniak, D. F., Nardi, A., Olney, J. W., Sartorius, L. & Holtzman, D. M. (2001) Exp. Neurol. 170, 326–344. [DOI] [PubMed] [Google Scholar]
- 56.Ferreira, S., Dupire, M.-J., Delacourte, A., Najib, J. & Caillet-Boudin, M.-L. (2000) Exp. Neurol. 166, 415–421. [DOI] [PubMed] [Google Scholar]
- 57.Poirier, J., Hess, M., May, P. C. & Finch, C. E. (1991) Mol. Brain Res. 11, 97–106. [DOI] [PubMed] [Google Scholar]
- 58.Selkoe, D. J. (2001) Physiol. Rev. 81, 741–766. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt, S., Barcellos, L. F., DeSombre, K., Rimmler, J. B., Lincoln, R. R., Bucher, P., Saunders, A. M., Lai, E., Martin, E. R., Vance, J. M., et al. (2002) Am. J. Hum. Genet. 70, 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lacomblez, L., Doppler, V., Beucler, I., Costes, G., Salachas, F., Raisonnier, A., Le Forestier, N., Pradat, P.-F., Bruckert, E. & Meininger, V. (2002) Neurology 58, 1112–1114. [DOI] [PubMed] [Google Scholar]
- 61.Zareparsi, S., Camicioli, R., Sexton, G., Bird, T., Swanson, P., Kaye, J., Nutt, J. & Payami, H. (2002) Am. J. Med. Genet. 107, 156–161. [DOI] [PubMed] [Google Scholar]