Abstract
Borrelia burgdorferi (Bb), the agent of Lyme disease, exists in nature through a complex enzootic life cycle that involves both ticks and mammals. As Bb transitions between its two diverse niches, profound adaptive changes occur that are reflected in differential patterns of gene expression, particularly involving lipoprotein genes. Using a mutagenesis approach, we show that Rrp2 (gene BB0763), one of the proteins predicted by the Bb genome (www.tigr.org) to be a response regulator of a two-component sensory transduction system, is a pivotal regulator governing the expression of major membrane lipoproteins such as OspC, DbpA, and Mlp8, as well as many other mammalian infection-associated immunogens of Bb. Sequence analysis additionally suggested that Rrp2 is a bacterial enhancer-binding protein, essential for σ54-dependent gene activation. Mutagenesis of a key amino acid residue within a putative activation domain revealed that Rrp2 controlled lipoprotein expression by governing the expression of the alternative σ-factor σs in a σ54-dependent manner. We therefore propose a signal transduction pathway involving Rrp2, σ54, and σs, which in concert control the expression of key lipoproteins and other infection-associated immunogens in Bb.
Borrelia burgdorferi (Bb), the spirochetal agent of Lyme disease, is maintained in nature through a complex enzootic life cycle that involves both tick and mammalian hosts (1). As Bb transitions between these two diverse environmental niches, profound adaptive responses occur, which are reflected in differential patterns of gene expression, the paradigm of which is the reciprocal regulation of outer surface (lipo)protein A (OspA) and outer surface (lipo)protein C (OspC) (2–6). These adaptive responses ostensibly are under the control of global regulatory mechanisms yet to be identified and characterized. In this regard, genome sequence information thus far has predicted relatively few potential global regulators in Bb (7). Among these are two alternative σ factors, sigmaN (RpoN; σN; σ54) and sigmaS (RpoS; σs; σ38) (8, 9). Studies on these two σ factors (10) have led to the discovery of the first regulatory (RpoN–RpoS) pathway in Bb, wherein σ54 controls the expression of σs which, in turn, regulates the expression of key membrane lipoproteins.
Among those membrane lipoproteins regulated by the RpoN–RpoS pathway are OspC and decorin-binding protein A (DbpA). Although the precise function of OspC is unknown, its up-regulation during tick feeding suggests that it facilitates Bb migration to tick salivary glands and/or spirochete transmission into mammalian tissues (5, 6, 11). DbpA is purported to facilitate the adherence of Bb to extracellular matrix as the spirochete invades mammalian dermal tissues (12–14). Recent evidence (15–17) suggests that the expression of other lipoproteins (denoted as group I lipoproteins), such as those within the multicopy lipoprotein (Mlp) paralogous family, are also influenced by the RpoN–RpoS pathway. Thus, the RpoN–RpoS pathway appears to play a prominent role in regulating a number of membrane lipoproteins associated with borrelial host adaptation and/or virulence expression.
Despite the differential expression of Bb membrane lipoproteins under various environmental conditions (2–6, 16, 18–21), virtually nothing has been known about signal transduction processes that govern differential lipoprotein expression in Bb. In this regard, two-component systems are a mainstay of signal transduction pathways in bacteria (22). Such two-component systems are typically comprised of a histidine kinase sensor and a downstream response regulator (22). The Bb genome is predicted to encode two putative two-component response regulators, Rrp1 (gene BB0419) and Rrp2 (gene BB0763) (7). Herein, we provide experimental evidence that Rrp2 governs the expression of OspC, DbpA, Mlp8, and many other Bb immunogens, and that Rrp2 serves as the activator for the RpoN–RpoS regulatory pathway. The combined findings provide insights into the regulatory mechanism(s) by which Bb modulates key features of its differential expression of membrane lipoproteins involved in Bb's complex life cycle and parasitic strategy.
Materials and Methods
Bacterial Strains and Growth Conditions. Bb strains used in this article are summarized in Table 1. An infectious clone of Bb, BbAH130, was recovered after plating 297 (Bb strain 297) (23) on Barbour–Stoenner–Kelly (BSK) agar medium. The clone was then needle-inoculated into mice and recovered from cultures of ear-punch biopsies. BbAH130 retains all 21 of the parental plasmids (determined by PCR; ref. 24), and is infectious for mice (unpublished data). rpoN or rpoS mutants, as well as a rpoN-complemented strain of Bb 297 were described (10). Borreliae were cultivated in vitro in BSK-H liquid medium (Sigma; ref. 25) under various environmental conditions. Conditions for adaptation of Bb to various culture temperatures were described (16). For all protein analyses, spirochetes were harvested at the late-logarithmic stage (ca. 3 × 107 cells per ml). Escherichia coli strain TOP 10 (Invitrogen) was used as a cloning host. E. coli strain BL21 was used as the host for recombinant protein expression and purification.
Table 1. Bb strains used in this article.
| Strain name | Purpose | Ref(s). |
|---|---|---|
| BbAH130 | Parental strain of an infectious clone of Bb strain 297 | This article |
| RpoSmut | rpoS mutant of Bb strain 297 | 10 |
| RpoNmut | rpoN mutant of Bb strain 297 | 10 |
| Rrp2G239C-Ermr | Clone with the rrp2 point mutation (G239C) linked to ermC | This article |
| Rrp2wt-Ermr | Clone harboring wild-type rrp2 linked to an ermC gene | This article |
| Rrp2wt-Strepr | A wild-type rrp2 allele was restored in the Rrp2G239C-Ermr mutant | This article |
| Rrp2G239C-Strepr | Clone with the rrp2 point mutation (G239C) linked to aadA | This article |
| Rrp2-G239C-Ermr + PflgB-rpoS | Clone Rrp2G239C-Ermr transformed with shuttle vector pALH227 that contains a constitutive rpoS driven by the flgB gene promoter of Bb | This article, 10 |
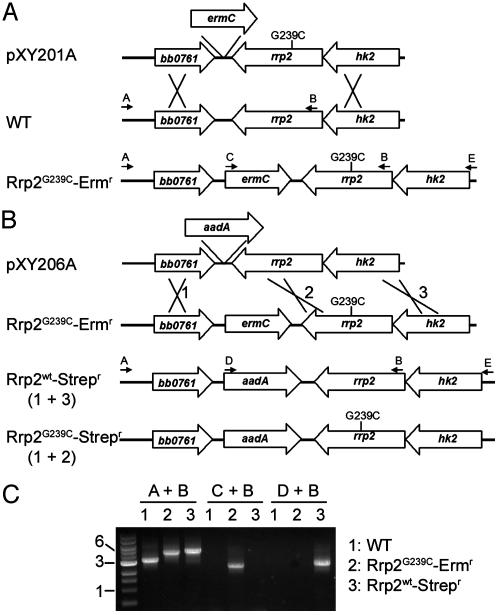
Construction of Suicide Plasmids. All recombinant DNA experiments and the use of antibiotic resistance markers in strain 297 were reviewed and approved by the University of Texas Southwestern Medical Center Biological and Chemical Safety Advisory Committee. A 5-kb DNA fragment containing rrp2 (gene BB0763) with flanking sequence was obtained by PCR amplification from 297 genomic DNA. PCR was performed by using the Expand High-Fidelity PCR system (Roche Diagnostics). The primer design was based on published gene sequences for Bb strain B31 (ref. 7 and primers A and E; see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org). The PCR fragment (GenBank accession no. AY309266) was then cloned into the pCR-XL-TOPO cloning vector (Invitrogen), resulting in plasmid pXY155. A BamHI site was then created downstream of rrp2 on pXY155 by using the QuikChange site-directed mutagenesis kit (Stratagene; primers F and G, Table 2). An ermC (from pJRS233) or aadA cassette [which confers streptomycin (Strep) resistance to Bb] from pKFSS1 (provided by S. Samuels, University of Montana, Missoula) was then amplified and the PCR fragment was inserted at the BamHI site, resulting in plasmid pXY212 and pXY206A (Fig. 2 A), respectively. Experiments revealed that the cassettes in either orientation relative to rrp2 caused recombinants to behave similarly. To create the Rrp2G239C mutation within the putative activation domain of Rrp2, a single-nucleotide change was created at the respective position in rrp2 on plasmid pXY212 by using the QuikChange site-directed mutagenesis kit (primers H and I; Table 2); this change resulted in plasmid pXY201A (Fig. 2 A). In pXY201A, the distance between the mutation site and the marker was ≈1 kb, which was flanked by ≈2 kb of additional Bb DNA on each side.
Fig. 2.
Strategy for mutagenesis of rrp2.(A) Generation of a point mutation within rrp2. Suicide plasmid pXY201A, encoding a point mutation corresponding to G239C in rrp2 linked to ermC, was transformed into Bb. On homologous recombination, strain Rrp2G239C-Ermr was generated. Crosses denote approximate positions of recombination (double crossover) events. Small arrows with letters denote positions and oligonucleotide primers used for PCR analyses. (B) Restoration of wild-type rrp2 in Rrp2G239C-Ermr. Suicide plasmid pXY206A, encoding wild-type rrp2 linked to aadA, was transformed into Rrp2G239C-Ermr. On homologous recombination, 80% of clones (denoted as Rrp2wt-Strepr) obtained the wild-type rrp2 allele after crossovers at positions 1 and 3. The other 20% of clones (denoted as Rrp2G239C-Strepr) occurred as a result of crossovers at positions 1 and 2, and thus these clones retained the mutant version of rrp2 linked to aadA. (C) PCR analysis of rrp2 transformants. Letter combinations denote primer pairs used for PCR. The lane at the left is a kb ladder.
Generation of the Rrp2G239C Bb Mutant. BbAH130 was made electrocompetent, and was transformed as described (10, 26). Colonies on pBSK agar medium (26) containing erythromycin (Erm) (50 ng/ml) appeared 2 weeks after plating. To confirm marker exchange, PCR was performed on whole-cell lysates of transformants by using the amplification strategy shown in Fig. 2 A (arrows). PCR fragments amplified with primers A and B were then subjected to DNA sequence analysis to verify the presence of the mutation corresponding to G239C. The resulting mutant was designated Rrp2G239C-Ermr (Fig. 2 A). To restore a wild-type rrp2 allele in this mutant, Rrp2G239C-Ermr was transformed with pXY206A. Clones resistant to 50 μg/ml Strep (Strepr clones) were verified (by PCR) to contain the correct aadA marker insertion, and the pertinent amplicon was DNA-sequenced to distinguish those clones that contained the wild-type copy of rrp2. A clone in which the wild-type rrp2 was successfully restored was designated as Rrp2wt-Strepr (Fig. 2B), and a clone that incorporated aadA, but retained the mutant rrp2 gene, was designated Rrp2wt-Strepr (Fig. 2B).
Antibodies, Antisera, and Protein Analyses. Rat polyclonal antisera, as well as monoclonal antibodies (14D2-27 and 8H3-33) directed against OspC, DbpA, Mlp8, σs, P6.6, OspA, or FlaB were reported (16, 27, 28). Sera collected from C3H/HeJ mice postinfestation with Ixodes scapularis nymphs harboring wild-type 297 were described (15); for the purposes of this article, sera collected 2–16 weeks postinfestation of these mice were pooled and used as a single reagent.
To generate polyclonal antiserum directed against the Bb Rrp2 protein, a DNA fragment encoding full-length Rrp2 was amplified by PCR from 297 genomic DNA (primers J and K; Table 2). The PCR product was then restriction enzyme-digested and ligated into the corresponding polylinker sites of pPROEX-Htb (BRL). After cloning and expression in E. coli, the N-terminal His6-tagged fusion protein was purified by affinity chromatography on a nickel-nitrilotriacetic acid matrix according to the manufacturer's instructions (Qiagen, Valencia, CA). Rat polyclonal antisera directed against Rrp2 was obtained by immunizing rats with the recombinant Rrp2 fusion protein according to published protocols (27). SDS/PAGE and immunoblotting were carried out as described (15); total protein from ≈5 × 107 spirochetes was loaded per gel lane.
RT-PCR. Total RNA from wild-type BbAH130 or mutants was isolated by using the NucleoSpin RNA II purification kit (BD Biosciences Clontech, Palo Alto, CA), according to instructions provided by the manufacturer. RT-PCRs for amplification of rpoS and ospC transcripts were performed by using ≈50 ng of total RNA, pertinent oligonucleotide primers, and the Titan One tube RT-PCR system (Roche Diagnostics; ref. 10).
Results
Strategy for Mutating rrp2 in Bb. Rrp2 has been predicted to be a response regulator in Bb (7), but the putative input signal and the potential output function(s) of Rrp2 have remained undefined. As a first approach toward elucidating the function of Rrp2, we attempted to disrupt the rrp2 gene. However, multiple attempts were unsuccessful, suggesting that Rrp2 might be essential for cell survival.
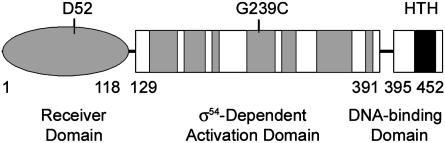
Further blast searches revealed that Rrp2 could be divided into three putative functional domains: an N-terminal receiver domain typical of a two-component response regulator, a central activation domain reminiscent of a σ54-dependent activator, and a C-terminal helix–turn–helix (HTH) motif, which is characteristic of a DNA-binding domain (Fig. 1). That the central domain of Rrp2 contains all seven motifs conserved among other σ54-dependent activators (ref. 29, Fig. 1, and Fig. 9, which is published as supporting information on the PNAS web site), suggests that in response to receiving sensory information, Rrp2 regulates the transcription of σ54-dependent genes.
Fig. 1.
Predicted domain structure for Bb Rrp2. The numbers denote amino acid residues. D52 is a putative phosphorylation site. The seven shaded boxes in the central domain represent motifs conserved among σ54-dependent activators (EBPs). G239C denotes the altered amino acid residue in the rrp2 mutant. HTH, helix–turn–helix motif.
It is well documented that to induce σ54-mediated transcription in bacteria, an essential activator, also known as an enhancer-binding protein (EBP), first must bind to an enhancer region upstream of where the Eσ54 holoenzyme is bound (30, 31). The inactive form of the EBP then must be activated (e.g., through phosphorylation), whereupon it catalyzes ATP hydrolysis and interacts with Eσ54 to result in transcription. Point mutations within the ATP-binding motifs (C1 or C4; ref. 29) of the central activation domain of EBPs typically abolishes their activation ability (loss of ATP-binding/hydrolysis), whereas leaving functions engendered by their other domains (e.g., phosphorylation, DNA binding) intact (32). This feature was exploited as a potential means of circumventing our prior inability to obtain rrp2 mutants, and to test the prediction that Rrp2 serves as a EBP in Bb. It was envisioned that obtaining such a mutant would enable us to elucidate the putative activation function of Rrp2 while, at the same time, avoiding lethality. To this end, we first performed in vitro site-directed mutagenesis to create a point mutation corresponding to G239C within the C4 ATP-binding motif of Rrp2 (Fig. 1). An ermC marker then was inserted downstream of the rrp2 gene (pXY201A; Fig. 2A). After transformation of Bb with this suicide plasmid, >30 Ermr recombinants were recovered containing the correct ermC insertion (verified by PCR; Fig. 2C). PCR fragments amplified by using primers A and B (Table 2 and Fig. 2 A) from 10 random clones were then chosen for DNA sequencing. All 10 clones (denoted as Rrp2G239C-Ermr; Fig. 2 A) harbored the desired mutation and the ermC marker. One clone was then selected for further sequence analysis of an amplicon obtained by using primers A and E (Fig. 2 A and Table 2); data revealed that the genes flanking rrp2 remained intact.
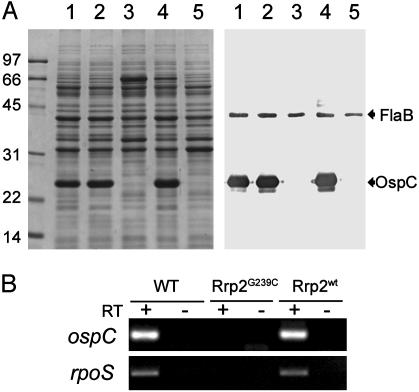
Mutation in the Activation Domain of Rrp2 Abolishes the Expression of OspC. A prominent phenotype of the Rrp2G239C-Ermr mutant was the absence of OspC expression, as revealed by both SDS/PAGE and immunoblot analyses (Fig. 3A, lanes 3). The abrogation of OspC expression in the Rrp2G239C-Ermr mutant was verified at the mRNA level by RT-PCR (Fig. 3B). The lack of OspC expression was not due to the insertion of the ermC gene within the Bb chromosome, inasmuch as a transformant with ermC linked to wild-type rrp2 retained normal OspC expression (Fig. 3A, lanes 2).
Fig. 3.
(A) SDS/PAGE (Coomassie blue stain) (Left) and immunoblot (Right) of whole-cell lysates of Bb strains. Numbers at left denote protein molecular mass markers in kDa. For immunoblotting, antibodies directed against FlaB and OspC were pooled. Strain designations are as in Table 1. Lanes 1, wild-type Bb; lanes 2, Rrp2wt-Ermr; lanes 3, Rrp2G239C-Ermr; lanes 4, Rrp2wt-Strepr; lanes 5, Rrp2G239C-Strepr. (B) RT-PCR analysis for ospC and rpoS in various strains (designated at the top): WT, wild-type 297; Rrp2G239C, Rrp2G239C-Ermr; and Rrp2wt, Rrp2wt-Strepr. RT, presence (+) or absence (–) of reverse transcriptase in RT-PCRs.
To verify that the lack of OspC expression in the Rrp2G239C-Ermr mutant was due solely to the mutation, the mutant was transformed with a suicide vector (pXY206A; Fig. 2 B) containing a wild-type rrp2 gene linked to a aadA marker (conferring Strep resistance to Bb). After homologous recombination, ≈25 resulting Strepr/Erms clones were obtained, and all clones yielded PCR amplification patterns that were consistent with the desired aadA insertion (Fig. 2 B and C). Further DNA sequencing revealed that 80% of the transformants (designated Rrp2wt-Strepr; Fig. 2B) incorporated a wild-type rrp2 gene in place of the mutant allele. In these transformants, OspC expression was fully restored (Fig. 3A, lanes 4). Such restoration was not due to the presence of aadA marker alone, inasmuch as those 20% of Strepr/Erms clones that recombined the aadA marker, but not the wild-type rrp2 gene (designated Rrp2G239C-Strepr; Fig. 2B), still did not express OspC (Fig. 3A, lanes 5).
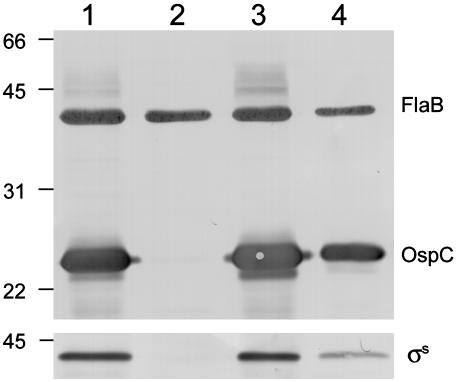
Rrp2 Controls the Expression of OspC Through σs. Experiments above indicated that the activation function of Rrp2 was essential for OspC expression. We reported earlier (10) that OspC expression also is controlled by the RpoN–RpoS regulatory pathway; in this pathway, σ54 is responsible for the production of σs which, in turn, initiates ospC transcription. Given that Rrp2 was predicted to be an EBP that activates σ54-dependent genes, it thus was reasonable to examine whether Rrp2 also controlled the expression of σs. As reported (10) for rpoN and rpoS mutants, expression of σs was abrogated in the Rrp2G239C-Ermr mutant strain (Fig. 4, lane 2). Restoring the rrp2 mutant with a wild-type copy of rrp2 (Rrp2wt-Strepr) rescued the expression of σs (Fig. 4, lane 3). In both instances, protein expression data (Fig. 4) were consistent with the absence or presence, respectively, of mRNAs detectable by RT-PCR (Fig. 3B). To further corroborate the idea that Rrp2 inf luenced OspC expression through σs, the Rrp2G239C-Ermr mutant was then transformed with a shuttle vector harboring a wild-type copy of rpoS driven by the constitutive Bb flgB promoter (PflgB) (10). In this strain, σs and OspC both were readily detectable by immunoblot (Fig. 4, lane 4), indicating that Rrp2 regulates the expression of OspC through its control of σs.
Fig. 4.
Immunoblot of Bb strains. (Upper) The panel was probed with a mixture of antibodies against FlaB and OspC. (Lower) The panel was probed with antibodies against σs. Strains used in lanes 1–3 are denoted as in Table 1. Lane 1, wild-type Bb; lane 2, Rrp2G239C-Ermr mutant; lane 3, a wild-type rrp2 allele was restored in the Rrp2G239C-Ermr mutant (Rrp2wt-Strepr); lane 4, the Rrp2G239C-Ermr mutant containing a shuttle plasmid encoding the constitutive expression of rpoS.
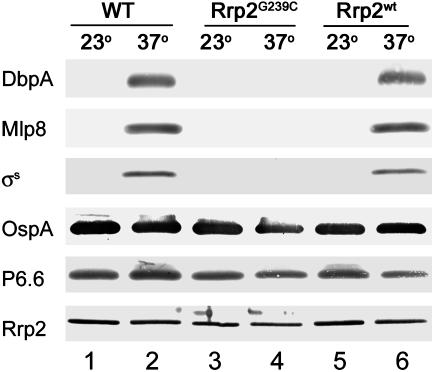
Other Lipoprotein Genes Controlled by Rrp2. OspC, σs, DbpA, and Mlp8 have been denoted as group I proteins, which are up-regulated in spirochetes growing at elevated temperature (37°C), reduced pH (6.8), or increased cell density (16). Given Rrp2's influence on OspC and σs expression, we examined whether Rrp2 similarly influenced the expression of other group I proteins. As shown (16), DbpA, Mlp8, and σs all were up-regulated in wild-type spirochetes cultivated at 37°C (Fig. 5, lane 2). In contrast, neither DbpA nor Mlp8 was detectable in the Rrp2G239C-Ermr mutant, regardless of culture temperature (Fig. 5, lanes 3 and 4). The clone in which the rrp2 mutation was restored with wild-type rrp2 (Rrp2wt-Strepr) regained temperature-induced DbpA and Mlp8 expression (Fig. 5, lane 6). Moreover, as in the case for OspC (Fig. 4, lane 4), rpoS driven by the pflgB promoter in the rrp2 mutant also restored the expression of DbpA and Mlp8 (data not shown). As predicted, levels of OspA and Lp6.6, lipoproteins that are down-regulated during mammalian infection (group II proteins) (2, 3, 6, 16, 27), were not affected in the Rrp2G239C-Ermr mutant (Fig. 5).
Fig. 5.
Immunoblots of Bb strains grown at various temperatures (°C). Antibodies used to detect the respective proteins are indicated on the left. Strains used are labeled at the top as in Table 1. WT, wild-type; Rrp2G239C, the Rrp2G239C-Ermr mutant; Rrp2wt; the Rrp2wt-Strepr strain.
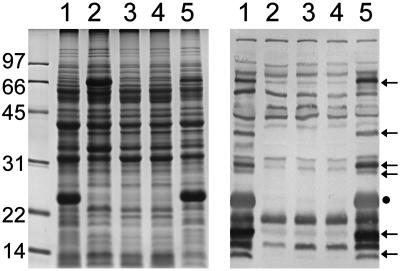
Infection-Associated Immunogen Profile of the rrp2 Mutant Differs Markedly from That of Wild-Type Bb. As an initial assessment of a role for Rrp2 in the global regulation of other mammalian infection-associated immunogens, whole-cell lysates of wild-type 297 or the Rrp2G296C-Ermr mutant were subjected to immunoblotting by using pooled sera collected from mice postinfestation with 297-infected I. scapularis nymphs. As shown in Fig. 6, markedly fewer infection-associated immunogens were detected within cell lysates of the Rrp2G296C-Ermr mutant (lane 2), as opposed to the wild-type (lane 1). Replacement of the mutant rrp2 allele with a wild-copy of rrp2 (Rrp2wt-Strepr) essentially restored the immunogen profile of the mutant to that of parental 297 (Fig. 6, lanes 5).
Fig. 6.
SDS/PAGE (Coomassie blue stain) (Left) and immunoblot (Right) of Bb strains. Numbers at the left denote protein molecular mass markers in kDa. For immunoblotting, pooled antisera were used from mice infected with Bb 297 by means of a tick bite. Lanes 1, wild-type Bb; lanes 2, Rrp2G239C-Ermr mutant; lanes 3, rpoN mutant; lanes 4, rpoS mutant; lanes 5, a wild-type rrp2 allele was restored in the Rrp2G239C-Ermr mutant (Rrp2wt-Strepr). Arrows denote antigenic proteins not expressed in the rrp2, rpoN, or rpoS mutants. The dot at the right indicates OspC.
To gain further insight into additional potential overlapping control of protein expression in Bb by all three global regulators Rrp2, σ54, and σs, the immunogen profiles of the three mutants were also compared. Although the immunogen profiles of the rpoN and rpoS mutants also differed dramatically from that of wild-type 297 (Fig. 6, lanes 3 and 4), they were strikingly similar to that of the Rrp2G239C-Ermr mutant (Fig. 6, lanes 2, 3, and 4).
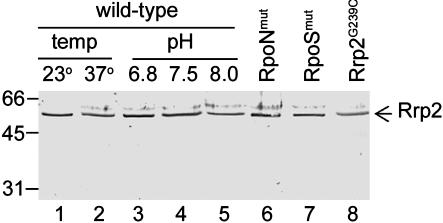
Rrp2 Is Constitutively Expressed in Bb. The combined data above support the contention that Rrp2 is a σ54-dependent activator that controls the expression of σs, which, in turn, controls the expression of other lipoproteins. Because it is common that σ54-dependent activators autoregulate their own expression by amplification in response to environmental stimuli (33), the expression of rrp2 in various Bb mutants was examined. As shown in Figs. 5 and 7 (lanes 6–8), Rrp2 levels did not differ among wild-type or any mutants deficient in either Rrp2, σ54, or σs. Given that genes controlled by Rrp2 are responsive to cultivation temperature and pH (10, 16), Rrp2 levels also were examined in wild-type 297 cultivated under these differing parameters. Rrp2 levels were not affected by any of the differing culture conditions tested (Figs. 5 and 7), suggesting that rrp2 is constitutively expressed in Bb.
Fig. 7.
Rrp2 is constitutively expressed by Bb under various culture temperatures, at various pH levels, and in various genetic backgrounds. Immunoblotting was used to detect Rrp2. Lanes 1–5, wild-type Bb; lanes 6 and 7, the rpoN mutant and the rpoS mutant (Table 1), respectively; lane 8, Rrp2G239C represents the Rrp2G239C-Ermr mutant.
Discussion
A significant challenge facing the postgenomic era is to ascribe actual functions to proteins of predicted annotated genes (34). In our initial efforts to elucidate signal transduction pathways that allow Bb to accommodate its complex life cycle, genetic studies described herein show an important regulatory role for Rrp2; one of two two-component response regulators predicted to be present in Bb (7). It was found that Rrp2 controlled the expression of a number of differentially regulated borrelial lipoproteins, including OspC, DbpA, and Mlp8, as well as many other mammalian infection-associated immunogens. Moreover, Rrp2 exhibits its control over OspC and other lipoproteins via the described RpoN–RpoS regulatory pathway (10).
An initial technical obstacle to studying Rrp2 function was the inability to generate Rrp2-deficient mutants after >10 attempts by using various transformation conditions, markers, and insertion sites. As such, we hypothesized that a gross defect in Rrp2 may be lethal for Bb. Bioinformatics performed by we and others (8, 35, 36) revealed that Rrp2 had a predicted central activation domain with homology to other σ54-dependent activators known as EBPs. To induce σ54-mediated transcription, an essential EBP must first bind to an enhancer region ≈100–1,000 base pairs upstream of where the Eσ54 holoenzyme is bound (at a distinctive –24/–12 promoter) (30, 31). The inactive form of the EBP must then be activated (e.g., through phosphorylation), whereupon it subsequently catalyzes ATP hydrolysis (the EBP is an ATPase), and interacts with the Eσ54 holoenzyme to result in open-complex formation (culminating in transcription) (30, 31). Given that rpoN mutations are not lethal in Bb (10), we postulated that a mutation in Rrp2 targeting only its σ54 activation function also should not be lethal. To accomplish this objective, we first created a point mutation in vitro in the rrp2 DNA sequence putatively encoding ATP binding (C4 motif) (Fig. 9 and ref. 29). Although a C1 ATP binding motif also is predicted in Rrp2 (Fig. 9 and ref. 29), the C4 motif is closer in proximity to the 3′ end of rrp2. The mutation was created at G239, a conserved residue shown in other systems to be essential for ATP binding (32). The defective rrp2 gene was then linked to an antibiotic resistance marker (ermC). On homologous recombination by means of a double crossover event, we predicted that some of the Ermr clones should acquire the rrp2 mutation from the suicide vector. In fact, all 10 random Ermr clones tested contained the desired mutation. Similarly, when we attempted to restore a wild-type copy of rrp2 into one of these Rrp2G239C-Ermr mutants, 80% of Strepr recombinants acquired wild-type rrp2. This mutagenesis strategy therefore appears to be relatively efficient, and thus should be broadly applicable to studying the functions of other Bb genes.
At least two lines of evidence from this article support the idea that Rrp2 is an EBP for σ54-dependent gene activation in Bb. First, a single amino acid change within the putative activation domain of Rrp2 abolished the expression of rpoS, a known σ54-dependent gene (10). Second, both the rrp2 and rpoN mutants displayed virtually identical immunogen profiles when they were immunoblotted with sera from Bb-infected mice, indicating that Rrp2 and σ54 control similar genes. We therefore conclude that Rrp2 is a σ54-dependent activator that works in concert with σ54 to control the expression of σs and other genes in Bb. Furthermore, inasmuch as the immunogen profile of the rpoS mutant mirrored those of the rrp2 and rpoN mutants, it appears that rpoS is a major downstream target of control by σ54. In addition, the control of σs by σ54 and Rrp2 likely is direct, inasmuch as computer analysis predicts a perfect –24/–12 σ54 promoter upstream of rpoS (8, 37).
Because we were unable to obtain rrp2-disrupted mutants, we postulated that in addition to serving as a σ54-dependent activator (EBP), Rrp2 may have other as yet undefined functions associated with Bb's survival. Rrp2 likely is a DNA-binding protein, and thus one such putative function would be to repress other borrelial genes whose expression otherwise is deleterious, at least under the in vitro cultivation conditions tested herein. Further studies will be necessary, however, to investigate other putative functions for Rrp2 in Bb.
Although Rrp2 appears to control σs and other lipoproteins differentially regulated by temperature in Bb, which is consistent with previous microarray data (38), Rrp2 was constitutively produced in spirochetes cultivated at 23°C or 37°C (Fig. 7). This finding would appear to be counterintuitive, inasmuch as σs and other proteins (e.g., OspC) are not expressed when Bb is cultivated at 23°C. One possibility in explaining this paradox is that although Rrp2 is expressed at 23°C, it is not activated. This explanation is logical, given that Rrp2 is a putative response regulator that ostensibly is activated only after phosphorylation. That is, Rrp2 may not be phosphorylated at 23°C, and temperature shift to 37°C may lead to phosphorylation and subsequent activation. An alternative explanation is that at either temperature, Rrp2 is both expressed and activated (phosphorylated; i.e., is capable of activating σs at 23°C), but at the 23°C condition there is an additional layer of regulation to counteract Rrp2-mediated activation of σs. The implication of this scenario is that Rrp2 may not regulate gene expression in a temperature-dependent manner, but rather in response to some other as yet unknown environmental signal(s). Critical to understanding how Rrp2 is activated is predicated ultimately on further identification and characterization of Rrp2's cognate histidine kinase (Hk; which is responsible for sensing the pertinent environmental stimulus). In this regard, the Bb gene immediately upstream of rrp2 is predicted to encode a Hk (BB0764) (7). Computer analysis further predicts that BB0764 and rrp2 are operonic (www.tigr.org). Therefore, the bb0764 gene product is the primary candidate Hk for Rrp2 and, as such, we herein designate BB0764 as Hk2. Interestingly, Galperin et al. (39) reported that the BB0764 (Hk2) also has a PAS domain; PAS domains typically bind organic molecules that are sensitive to oxygen tension, light, redox potentials, small ligands, and overall energy states of the cell (40). This result raises the provocative possibility that activation of Rrp2 ultimately occurs not in response to temperature per se, but rather in response to Hk2 receiving other sensory information such as oxygen tension, redox potential, etc. All of these intriguing possibilities remain to be more fully explored.
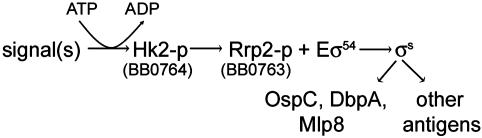
Data from earlier work (10) and this article, as well as the computer predictions for Hk2, allow us to propose a model in which Rrp2 ultimately controls the expression of key lipoproteins and other protein antigens in Bb (Fig. 8). Sensory information likely channels into the sensor domain of Hk2 and triggers Hk2 autophosphorylation at its relevant His residue (His-162 is the reasonable candidate site). Phosphorylated Hk2 then ostensibly phosphorylates Rrp2 at its conserved residue (Asp-52). With phosphorylation, Rrp2 becomes activated. The active form of Rrp2 then hydrolyzes ATP, interacts with Eσ54 holoenzyme, and activates transcription of σ54-dependent genes. rpoS is a principal target activated by this pathway, culminating in the expression of OspC, DbpA, Mlp8, and other infection-associated protein antigens of Bb. Although data provided in this article firmly demonstrate the control by Rrp2 over the expression of key Bb membrane lipoproteins, further experiments are warranted to investigate the molecular features of Hk2 and its putative relationship to Rrp2.
Fig. 8.
Proposed mechanism of Rrp2 control over the RpoN–RpoS regulatory pathway in Bb (see text).
Supplementary Material
Acknowledgments
We thank Martin Goldberg for assistance with purifying Rrp2 and generating Rrp2 antiserum, and Jon Blevins and Andrew Revel for critical review of the manuscript. This work was supported by Public Health Service Grant AI-45538 (to M.V.N.).
Abbreviations: Bb, Borrelia burgdorferi; 297, B. burgdorferi strain 297; Dbp, decorin-binding protein; EBP, enhancer-binding protein; Erm, erythromycin; Mlp, multicopy lipoprotein; Rrp, response regulator protein; Strep, streptomycin; Osp, outer surface protein; Hk, histidine kinase.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY309266).
References
- 1.Steere, A. C. (1993) Hosp. Pract. 28, 37–44. [DOI] [PubMed] [Google Scholar]
- 2.Schwan, T. G., Piesman, J., Golde, W. T., Dolan, M. C. & Rosa, P. A. (1995) Proc. Natl. Acad. Sci. USA 92, 2909–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery, R. R., Malawista, S. E., Feen, K. J. M. & Bockenstedt, L. K. (1996) J. Exp. Med. 183, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Silva, A. M., Telford, S. R., Brunet, L. R., Barthold, S. W. & Fikrig, E. (1996) J. Exp. Med. 183, 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohnishi, J., Piesman, J. & de Silva, A. M. (2001) Proc. Natl. Acad. Sci. USA 98, 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwan, T. G. & Piesman, J. (2000) J. Clin. Microbiol. 38, 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser, C. M., Casjens, S., Huang, W. M., Sutton, G. G., Clayton, R., Lathigra, R., White, O., Ketchum, K. A., Dodson, R., Hickey, E. K., et al. (1997) Nature 390, 580–586. [DOI] [PubMed] [Google Scholar]
- 8.Studholme, D. J. & Buck, M. (2000) FEMS Microbiol. Lett. 186, 1–9. [DOI] [PubMed] [Google Scholar]
- 9.Hengge-Aronis, R. (1999) Curr. Opin. Microbiol. 2, 148–152. [DOI] [PubMed] [Google Scholar]
- 10.Hubner, A., Yang, X., Nolen, D. M., Popova, T. G., Cabello, F. C. & Norgard, M. V. (2001) Proc. Natl. Acad. Sci. USA 98, 12724–12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilmore, R. D. & Piesman, J. (2000) Infect. Immun. 68, 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo, B. P., Norris, S. J., Rosenberg, L. C. & Hook, M. (1995) Infect. Immun. 63, 3467–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo, B. P., Brown, E. L., Dorward, D. W., Rosenberg, L. C. & Hook, M. (1998) Mol. Microbiol. 30, 711–723. [DOI] [PubMed] [Google Scholar]
- 14.Brown, E. L., Guo, B. P., O'Neal, P. & Hook, M. (1999) J. Biol. Chem. 274, 26272–26278. [DOI] [PubMed] [Google Scholar]
- 15.Yang, X., Popova, T. G., Hagman, K. E., Wikel, S. K., Schoeler, G. B., Caimano, M. J., Radolf, J. D. & Norgard, M. V. (1999) Infect. Immun. 67, 6008–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang, X., Goldberg, M. S., Popova, T. G., Schoeler, G. B., Wikel, S. K., Hagman, K. E. & Norgard, M. V. (2000) Mol. Microbiol. 37, 1470–1479. [DOI] [PubMed] [Google Scholar]
- 17.Yang, X., Hubner, A., Popova, T. G., Hagman, K. E. & Norgard, M. V. (2003) Infect. Immun. 71, 5012–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang, F. T., Nelson, F. K. & Fikrig, E. (2002) Infect. Immun. 70, 3300–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagman, K. E., Yang, X., Wikel, S. K., Schoeler, G. B., Caimano, M. J., Radolf, J. D. & Norgard, M. V. (2000) Infect. Immun. 68, 4759–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Indest, K. J., Ramamoorthy, R., Sole, M., Gilmore, R. D., Johnson, B. J. B. & Philipp, M. T. (1997) Infect. Immun. 65, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll, J. A., Garon, C. F. & Schwan, T. G. (1999) Infect. Immun. 67, 3181–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stock, A. M., Robinson, V. L. & Goudreau, P. N. (2000) Annu. Rev. Biochem. 69, 183–215. [DOI] [PubMed] [Google Scholar]
- 23.Norton Hughes, C. A., Kodner, C. B. & Johnson, R. C. (1992) J. Clin. Microbiol. 30, 698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eggers, C. H., Caimano, M. J., Clawson, M. L., Miller, W. G., Samuels, D. S. & Radolf, J. D. (2002) Mol. Microbiol. 43, 281–295. [DOI] [PubMed] [Google Scholar]
- 25.Pollack, R. J., Telford, S. R. & Spielman, A. (1993) J. Clin. Microbiol. 31, 1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels, D. S. (1995) in Methods in Molecular Biology, ed. Nickoloff, J. A. (Humana, Totowa, NJ), pp. 253–259.
- 27.Lahdenne, P., Porcella, S. F., Hagman, K. E., Akins, D. R., Popova, T. G., Cox, D. L., Katona, L. I., Radolf, J. D. & Norgard, M. V. (1997) Infect. Immun. 65, 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagman, K. E., Lahdenne, P., Popova, T. G., Porcella, S. F., Akins, D. R., Radolf, J. D. & Norgard, M. V. (1998) Infect. Immun. 66, 2674–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osuna, J., Soberon, X. & Morett, E. (1997) Protein Sci. 6, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wosten, M. M. S. M. (1998) FEMS Microbiol. Rev. 22, 127–150. [DOI] [PubMed] [Google Scholar]
- 31.Buck, M., Gallegos, M.-T., Studholme, D. J., Guo, Y. & Gralla, J. D. (2000) J. Bacteriol. 182, 4129–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, Y.-K. & Hoover, T. R. (1997) J. Bacteriol. 179, 5812–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reitzer, L. & Schneider, B. L. (2001) Microbiol. Mol. Biol. Rev. 65, 422–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauss, E. J. & Falkow, S. (1997) Science 276, 707–712. [DOI] [PubMed] [Google Scholar]
- 35.Studholme, D. J. & Dixon, R. (2003) J. Bacteriol. 185, 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramanian, G., Koonin, E. V. & Aravind, L. (2000) Infect. Immun. 68, 1633–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Studholme, D. J. & Buck, M. (2000) Microbiology 146, 4–5. [DOI] [PubMed] [Google Scholar]
- 38.Revel, A. T., Talaat, A. M. & Norgard, M. V. (2002) Proc. Natl. Acad. Sci. USA 99, 1562–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galperin, M. Y., Nikolskaya, A. N. & Koonin, E. V. (2001) FEMS Microbiol. Lett. 203, 11–21. [DOI] [PubMed] [Google Scholar]
- 40.Taylor, B. L. & Zhulin, I. B. (1999) Microbiol. Mol. Biol. Rev. 63, 479–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.