Abstract
Gamma (30–80 Hz) oscillations occur in mammalian electroencephalogram in a manner that indicates cognitive relevance. In vitro models of gamma oscillations demonstrate two forms of oscillation: one occurring transiently and driven by discrete afferent input and the second occurring persistently in response to activation of excitatory metabotropic receptors. The mechanism underlying persistent gamma oscillations has been suggested to involve gap-junctional communication between axons of principal neurons, but the precise relationship between this neuronal activity and the gamma oscillation has remained elusive. Here we demonstrate that gamma oscillations coexist with high-frequency oscillations (>90 Hz). High-frequency oscillations can be generated in the axonal plexus even when it is physically isolated from pyramidal cell bodies. They were enhanced in networks by nonsomatic γ-aminobutyric acid type A (GABAA) receptor activation, were modulated by perisomatic GABAA receptor-mediated synaptic input to principal cells, and provided the phasic input to interneurons required to generate persistent gamma-frequency oscillations. The data suggest that high-frequency oscillations occurred as a consequence of random activity within the axonal plexus. Interneurons provide a mechanism by which this random activity is both amplified and organized into a coherent network rhythm.
Rhythmic neuronal activity at gamma frequencies underlies aspects of cognitive function (1, 2). Persistent gamma oscillations (3) require interneurons to receive phasic bursts of excitatory postsynaptic potentials (EPSPs), but these are seen to occur with very low frequencies of principal cell somatic spiking (4). Blockade of gap junctions abolishes these EPSPs and the gamma oscillation. However, gamma-frequency oscillations still occur in the absence of gap junctions between interneuron dendrites (5), suggesting a specific role for proposed axonal gap junctions (6, 7). Given this relatively low pyramidal cell somatic spike rate (≈2 Hz), with respect to the frequency of EPSPs invading interneurons, the question remains as to how the large phasic drive to interneurons is generated. Clues to the answer of this question arise from work demonstrating collective activity in pools of axons as a consequence of ectopic action-potential generation in the axons of principal cells themselves. These ectopic action potentials are able to spread between neighboring axons via axo-axonic gap junctions. Irregularly occurring bursts of collective activity in principal cells are observed spontaneously (8) and are believed to be mediated by ectopic activity propagating between electrically and dye-coupled axons (6). Given the degree of convergence of principal cell input onto individual interneurons, it is feasible that this collective behavior, in the near absence of somatic, orthodromic spiking, could provide the drive observed to be necessary for the generation and maintenance of persistent gamma-frequency oscillations. However, to do this the axonal collective behavior must be generated by known mechanisms of gamma-frequency rhythmogenesis and must be modulated at gamma frequency to provide the appropriate, observed phasic drive to interneurons.
Here we demonstrate the coexistence of phasic, high-frequency oscillations in principal cell axon populations and field potential gamma-frequency oscillations in the kainate model of hippocampal rhythmic behavior. We demonstrate a reciprocal interaction between GABAA receptor activation and the generation and modulation of collective axonal behavior consistent with a principal cell axonal generator underlying persistent gamma-frequency field potential oscillations.
Materials and Methods
Experimental Methods. Transverse hippocampal slices (450-μm) were prepared from adult Sprague–Dawley rats in accordance with United Kingdom Home Office (London) guidelines. Slices of area CA1 were immediately cut to remove area CA3 and the subiculum. Half of the sections were then further cut along stratum oriens proximal to stratum pyramidale to produce minisections containing only stratum oriens and the alveus. Sections were transferred to a recording chamber and maintained at the interface between artificial cerebrospinal fluid (aCSF) (126 mM NaCl/3 mM KCl/1.25 mM NaH2PO4/24 mM NaHCO3/2 mM MgSO4/2 mM CaCl2/10 mM glucose) and 95% O2/5% CO2. Extracellular field recordings were taken by using glass micropipettes (resistance, 0.5–1 MΩ) filled with the aCSF described above. All drugs were obtained from Sigma and Tocris Cookson (Bristol, U.K.). Spectral analyses were performed by using matlab (Mathworks, Natick, MA).
Computer Modeling Methods. Two types of simulations were performed by using closely related computer programs. For simulating the “full” network (chemical synapses + electrical coupling between axons of pyramidal cells and between interneuron dendrites), we used the same program as described in ref. 7, with an interneuron gap junction conductance of 1.84 nS. To simulate the axonal plexus, we took this same program but blocked all chemical synapses; we also set to zero the conductance between axon initial segment and soma of all pyramidal neurons (electrical coupling between axons was retained). In each model axonal ectopic spikes were generated by 0.4-nA, 0.4-ms depolarizing current pulses to distal axonal compartments. Ectopic spike generation had, in each axon, Poisson statistics (using a pseudorandom number generator) with a prespecified mean rate; ectopic generation in different axons was statistically independent. Extracellular fields were represented as the inverted local average of 224 pyramidal cell somatic potentials. Local axonal average was represented as the average of nine local axon membrane potentials (at the site where gap junctions could form).
Results
In slices of rat hippocampal area CA1, bath application of kainate (1 μM) generated gamma-frequency oscillations (38 ± 7 Hz, n = 5). Smaller, high-frequency events (>100 Hz) were seen superimposed on the field gamma-frequency rhythm only in stratum oriens and pyramidale. These rapid events were phase-locked to the negative part of the stratum oriens field potential and occurred with a mean incidence of 0.82 ± 0.05 bursts per gamma period (five slices). Spectral analysis of high-pass-filtered data showed a clear pattern of activity over a broad frequency range (120–400 Hz) modulated at gamma frequencies (Fig. 1a). Field responses in stratum radiatum showed no high-frequency events (n = 5).
Fig. 1.
Coexistence of field gamma-frequency oscillations and high-frequency oscillations in hippocampal area CA1. (a) Gamma oscillations generated in a CA1 slice by 1 μM kainate. Field oscillations are seen in both stratum oriens (s. oriens) and stratum radiatum (s. rad.). High-pass filtering of the field recording (>100 Hz) reveals discrete epochs of high-frequency activity phase-locked to the ongoing gamma oscillation in stratum oriens but not stratum radiatum. Spectrograms of the high-pass-filtered data reveal bursts of activity within a broad frequency range modulated at gamma frequencies in stratum oriens only. (b) Stratum oriens and stratum radiatum recordings in the presence of 1 μM kainate in slices where stratum oriens and alveus are separated from stratum pyramidale and stratum radiatum. Kainate generated a near-continuous train of high-frequency activity in stratum oriens but no activity in stratum radiatum. Spectrograms of the high-pass-filtered data show a more narrow distribution of frequencies with no modulation at gamma frequencies. No activity was seen in stratum radiatum. (Scale bars, 0.2 mV and 100 ms.)
Similar, although randomly occurring, bursts of high-frequency oscillations occur in non-gamma-oscillating slices with only sparse recruitment of principal cell somata (8). These events were gap junction-dependent but could not arise from dendrodendritic coupling because both the kinetics and frequency of the coupling potentials were inconsistent with activity in dendritic compartments. Thus these events were predicted to arise from activity in the axons of principal cells coupled via axoaxonic gap junctions (6, 8, 9). To test this prediction we lesioned slices of area CA1 such that stratum oriens was separated from stratum pyramidale and stratum radiatum (Fig. 1b). In the absence of kainate these stratum oriens minisections still displayed random bursts of high-frequency oscillations (data not shown), indicating that their mechanism of generation did not involve the pyramidal cell somatodendritic compartments. Bath application of kainate (1 mM) to the minisections failed to generate gamma-frequency oscillations in either stratum radiatum or stratum oriens. However, high-frequency field activity was seen in the isolated stratum oriens. This activity took the form of near-continuous oscillation (frequency range, 110–190 Hz; mean, 148 ± 12 Hz; n = 5; Fig. 1b) and was insensitive to blockade of fast glutamatergic receptors (20 μM R-CPP and 20 μM SYM2206) and γ-aminobutyric acid (GABA) type B (GABAB) receptors (100 nM CGP55845). No such activity was seen in the minisections containing stratum pyramidale and stratum radiatum (n = 5).
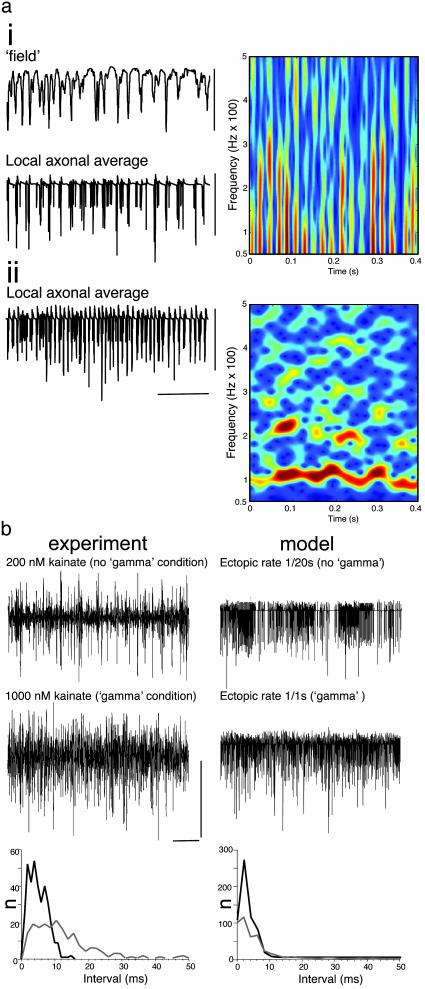
A computer model of the hippocampal cornu ammonis (4, 7), containing axo-axonic gap junctions to form an axonal plexus, generated gamma-frequency field oscillations with pseudorandom ectopic action-potential generation at modal frequencies of ≥1 per axon per s. Local axonal average (n = 9 axons) membrane potentials reproduced the experimental observations illustrated in Fig. 1a (Fig. 2a). Bursts of high-frequency oscillations were seen modulated at the field gamma frequency and occurring on the negative peak of the global average field potential. Furthermore, the model was manipulated to allow examination of the activity in the axonal plexus in the absence of chemical synapses, and also in the additional absence of pyramidal cell soma/dendrites, to mimic the experimental separation of stratum oriens from strata pyramidale and radiatum. This showed the same transition as seen in experiment: from gamma-modulated bursts of high-frequency activity (40 to >500 Hz) to tonic high-frequency activity with a clear modal frequency of 100 Hz (Fig. 2a).
Fig. 2.
Collective behavior in the axonal plexus depends on rate of ectopic spike generation: relationship to field gamma-frequency oscillations. (ai) Simulated gamma oscillation in the “intact” network (cf. ref. 7) with a mean ectopic rate of 1 per s per pyramidal axon. The “field” is the average somatic potential of 224 nearby pyramidal cells, and the “local axonal average” is the potential in midaxon of nine nearby pyramidal cell axons. Note the high-frequency components in the axonal average. (Right) Spectrogram taken from local axon average data. (aii) Simulated “isolated axonal plexus” (see Materials and Methods) with the same ectopic spike rate as shown in ai. (Right) Spectrogram from data. Compare with Fig. 1b. [Scale bars: ai, 10 mV; aii, 40 mV and 100 ms.] (b) Concentration dependence of high-frequency activity in the isolated oriens minisection and the model isolated plexus. (Left) Kainate (200 nM) fails to generate gamma-frequency oscillations in the intact CA1 minisection (data not shown) but does generate sporadic periods of high-frequency activity in these sections and in stratum oriens minisections (illustrated). In contrast, 1 μM kainate does generate gamma-frequency field oscillations in the intact CA1 section (see Fig. 1) and generates near-continuous high-frequency oscillations in the oriens minisection (illustrated). Plots of spike incidence in the stratum oriens field recordings revealed an increase in the number of spikes occurring with instantaneous frequencies of >100 Hz. The data shown are an example of spike incidence in a 2-s epoch of data from minisections in the presence of 200 nM (gray line) and 1 μM kainate (black line). (Right) Occurrence of random ectopic spikes with a modal incidence of 1 spike per axon per 20 s failed to generate field gamma-frequency oscillations in the intact model (data not shown) and induced sporadic high-frequency activity in the axonal plexus (illustrated as local axon average as shown in a). In contrast, an increased rate of ectopic spike generation (1 per axon per s) did generate field gamma oscillations (see a) and also generated a near-continuous presence of high-frequency activity in the isolated plexus. As with the experiment, this is accompanied by an increase in the incidence of spikes occurring with instantaneous frequencies >100 Hz. [Scale bars: Left, 0.2 mV and 200 ms; Right, 40 mV and 200 ms.]
In the stratum oriens minisection, kainate enhanced the occurrence of high-frequency activity in a concentration-dependent manner. However, continuous high-frequency activity was only seen at concentrations sufficient to generate field gamma oscillations in the intact CA1 slices (≥1 μM). Interspike intervals for the local axonal field showed a large increase in events occurring with instantaneous frequencies of ≥100 Hz (120 ± 15%, P < 0.05, n = 5) when increasing kainate concentration from 200 nM (which did not generate gamma-frequency field oscillations) to 1 μM (which did generate gamma-frequency field oscillations; Fig. 2b, compare with Fig. 1a). Similarly, the model axonal plexus demonstrated a large increase in activity within this frequency range when comparing activity induced by an ectopic rate of 1 per 20 s (not sufficient to generate gamma-frequency field oscillations) with activity induced by a rate of 1 per s (sufficient to generate field gamma oscillations).
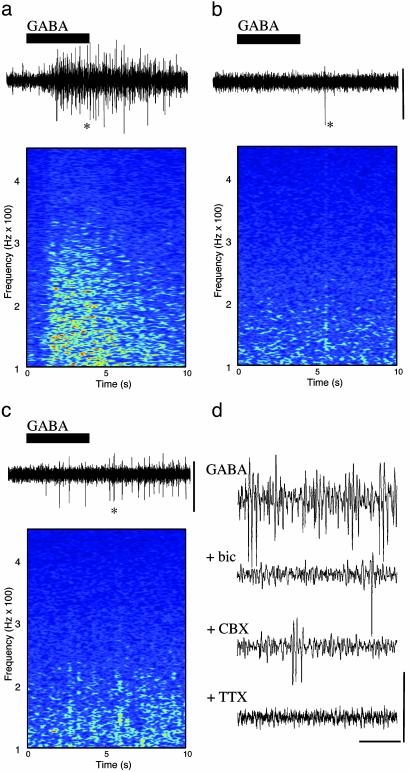
Kainate directly excites interneuronal axons generating GABA release (10). In the peripheral nervous system and spinal cord, GABA, acting via GABA type A (GABAA) receptors, has a direct excitatory effect on principal neuronal axons (11–13). In the hippocampus, ectopic action potentials generated at axonal sites distal to principal cell bodies were enhanced by GABAA receptor activation (14, 15). A 3-fold increase in ectopic action-potential generation by GABA application to axonal sites distal to the cell soma is seen in thalamic relay neurons (16). We tested the hypothesis that GABA release, acting on principal cell axons, is responsible for the large enhancement of axonal plexus activity in stratum oriens minisections in the presence of kainate. When GABA (0.5 mM) was applied, a large, transient increase in high-frequency activity was seen. Mean power (from 60 to 300 Hz) increased from 0.14 ± 0.03 μV2·Hz–2 to 2.2 ± 0.3 μV2·Hz–2 (P < 0.005, n = 5; Fig. 3a). This activity was blocked by the GABAA receptor antagonist bicuculline (20 μM; Fig. 3b) and the sodium channel blocker tetrodotoxin (2 μM; Fig. 3d) and significantly reduced by the gap junction blocker carbenoxolone (0.2 mM; P < 0.05, n = 4: Fig. 3c). Bicuculline blocked gamma-frequency oscillations (17, 18) and high-frequency oscillations coexistent with gamma oscillations (data not shown) and reduced the continuous high-frequency activity induced by kainate in stratum oriens minisections (power with kainate alone, 3.14 ± 0.11 μV2·Hz–2; kainate plus bicuculline, 0.5 ± 0.3 μV2·Hz–2; P < 0.05, n = 5).
Fig. 3.
GABA acting at GABAA receptors generates collective axonal behavior in a gap junction and action potential-dependent manner in stratum oriens minisections. (a) Stratum oriens minisections were bathed in aCSF containing 50 μM D-AP5 and 20 μM SYM 2206. This solution, containing in addition 0.5 mM GABA, was transiently perfused into the bath as illustrated (black bar). GABA generated a transient period of enhanced high-frequency activity as illustrated in the spectrogram. (b) Preperfusion of the stratum oriens minislices with 20 μM bicuculline abolished the effects of GABA perfusion. (c) Preperfusion with 0.2 mM carbenoxolone significantly reduced the GABA-induced collective axonal behavior. Time axes for the traces are the same as the spectrograms. (Scale bars, 50 μV.) (d) Expanded time axis recordings of the data illustrated in a–c (asterisks). Tetrodotoxin blocked all spontaneous activity and that induced by GABA. (Scale bars, 50 μV and 100 ms.)
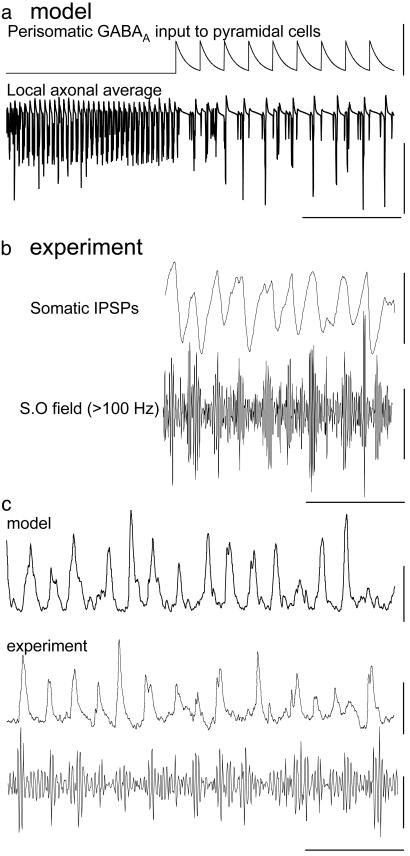
In the intact CA1 slice (containing both axonal and somatodendritic compartments) in the presence of 1 μM kainate, sufficient activity in the plexus existed to generate a field gamma-frequency oscillation. However, in the isolated plexus this activity takes the form of a near-continuous fast oscillation with frequency >100 Hz. Because perisomatic GABA application does not generate axonal spiking (15) these observations suggested that conventional inhibitory GABAergic effects, proximal to the somata, may play an additional role in phasing high-frequency, axonal plexus activity during a field gamma oscillation. To test this dual role of GABAA receptors we generated axonal plexus activity in the simulated network (chemical synapses blocked, ectopic activity of 1 per s per axon). We then introduced a 40-Hz train of extrinsic inhibitory postsynaptic potentials (IPSPs) to the perisomatic compartments of the model principal cells. This idealized model of perisomatic GABAA receptor-mediated inhibition generated phasic bursts of high-frequency activity similar to those seen in both the intact CA1 slice and the simulated network with chemical synaptic activity intact (Fig. 4 a and b; compare with Fig. 1). Therefore, synaptic release of GABA can both generate collective behavior in stratum oriens axons by acting at axonal sites distal to principal cell somata and provide the means to phase this collective behavior at gamma frequencies in a manner dependent on perisomatic inhibition.
Fig. 4.
Pattern of collective axonal behavior induced by kainate during gamma field oscillations is GABAA receptor-dependent: reciprocal interaction with interneurons. (a) Model data (intact network with recurrent synapses blocked) showing local axonal average in response to an ectopic rate of 1 per axon per s (compare with Fig. 2aii). During the initial part of the trace no perisomatic inhibition is present, and then a 40-Hz train of perisomatic inhibitory synaptic events was fed into the system. It should be noted that these events were generated exogenously, not as part of the collective behavior of the entire network. Perisomatic synaptic inhibition at 40 Hz modulated the collective axonal behavior in a manner closely resembling that seen in the intact model (Fig. 2ai). (Scale bars: Upper,40nS; Lower, 40 mV and 100 ms.) (b) Concurrent experimental recordings of somatic IPSP (from a membrane potential of –30 mV; Upper) and high-pass-filtered stratum oriens field (Lower). Note the occurrence of activity on the decay phase of IPSPs, terminated by the onset of the subsequent IPSP. S.O field, stratum oriens field. (Scale bars: Upper, 6 mV; Lower, 10 μV and 100 ms.) (c) Collective axonal behavior generates compound EPSPs in fast-spiking interneurons in CA1 stratum pyramidale during field gamma oscillations in the intact CA1 sections (cell impaled with electrodes containing 50 mM QX314 to block action potentials and hyperpolarized to –70 mV). Superimposed below is the concurrent stratum oriens field recording high-pass-filtered at 100 Hz. This pattern of behavior is identical to that seen in the intact model for total α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated input to an interneuron. Model ectopic rate, 1 per s per pyramidal cell axon. (Scale bars: Top, 4 nS; Middle, 5 mV; Bottom, 10 μV and 100 ms.)
In the intact network model and CA1 slice experiments, the resulting phasic bursts of high-frequency collective axonal activity resulted in compound EPSPs in fast-spiking stratum pyramidale interneurons (Fig. 4c), thus maintaining interneuronal action-potential generation and the persistent gamma-frequency field oscillation. Similar patterns of compound EPSPs, consisting of high-frequency barrages of EPSPs modulated at gamma frequency, were also seen in axo-axonic, oriens lacunosum moleculare and bistratified interneurons in cornu ammonis and in fast-spiking interneurons in the superficial medial entorhinal cortex during kainate-induced gamma-frequency field oscillations (data not shown).
Discussion
Our model and experiments indicate a specific relationship between activity within a plexus of gap-junctionally connected axons and persistent gamma oscillations as measured by field potential recordings. Previous work has demonstrated high-frequency activity in stratum pyramidale with intracellular correlates at the level of the pyramidal cell soma (8). However, the conclusion of this work was that the origin of high-frequency activity was axonal. Therefore separation of stratum oriens from stratum pyramidale would be expected to preserve this activity as indeed it did. Stratum oriens contains a small number of displaced pyramidal neurons, interneurons, and a large number of pyramidal cell basal dendrites in addition to the pyramidal cell axons. However, the activity was most likely to arise from the axons for a number of reasons. First, interneurons do not have the appropriate cellular morphology to generate fields of the amplitude seen. Second, the high-frequency activity seen was resistant to blockers of fast glutamatergic excitation, suggesting that a conventional, synaptic network of displaced pyramidal neurons could also not be responsible for the observed activity. Third, gap junctions have not been demonstrated between pyramidal basal dendrites in the adult rat hippocampal CA1. If gap-junctional connectivity existed between pyramidal cell dendrites, it would not be capable of generating such high-frequency events because of the cable properties of these neuronal compartments (8).
For the type of activity seen to be generated in a plexus of gap-junctionally connected axons, a low rate of nascent ectopic action-potential generation was predicted to be necessary. The actual rate of nascent ectopic spike generation was impossible to measure experimentally, because there would be no way to distinguish an action-potential generated in any given axon as a consequence of a local random event or transmitted from neighboring axons via gap-junctional connections. However, the computer model predicted that high-frequency activity could be generated over a wide range of ectopic spike generation frequencies from 1 per 20 s to 1 per s per axon, a very low value with respect to the frequencies observed from the collective behavior (>80 Hz). Collective behavior corresponding to this range of ectopic generation was seen with exogenous application of GABA (Fig. 3).
The pattern of activity exposed in conditions supporting a field gamma oscillation corresponded to an ectopic rate of 1 spike per s per axon in the model. This level of activity generated a near-continuous collective rhythm at modal frequencies ≈100–200 Hz in the isolated stratum oriens. In the intact slice this activity was broken up into small packets at gamma frequencies by a mechanism predicted to involve perisomatic GABAergic synapses. Even with somatic inhibition the present data and model support the notion of a large degree of activity percolating through an axonal network. Given that principal cells receive excitatory synapses from other principal cells, it is not clear why such behavior favors a local inhibition-based rhythm over recurrent excitation. The rate and amplitude of excitatory postsynaptic events in principal cells during kainate-induced gamma oscillations are at least 1 order of magnitude lower than in local interneurons (e.g., see ref. 5). However, collective activity within an axonal plexus would be expected to distribute across all principal cell synapses. The reason why collective axonal activity seems to favor activation of interneurons may be found in the relative efficacies of the two above-mentioned synapse types. CA1 recurrent pyramid–pyramid connectivity is in the order of 1%, with synapses on distal dendrites generating individual somatic EPSPs only of magnitude ≈1 mV (19). In contrast, pyramid–fast-spiking interneuron connectivity can be as much as 14%, with synapses targeting active dendrites and generating individual somatic EPSPs up to 4 mV in amplitude (20–22). Thus the local network seems to be “tuned” to respond to collective axonal activity by favoring synaptic activation of interneurons over principal cells.
Two levels of temporal organization of ongoing ectopic activity in the axonal plexus could be generated by GABA. First, if there is a sufficiently high level of random spike generation in individual axons and gap-junctional coupling between axons, then a high-frequency field oscillation results as a collective behavior of the plexus itself. This activity seemed to be generated via GABAergic activation of principal cell axons or axon terminals. Kainate has been shown to directly excite interneuronal axon and/or terminal compartments (10), leading to GABA release independently of somatic interneuronal activity. Second, the activity within the plexus converges on interneurons (in the model each receives input from 150 pyramidal neurons), generating interneuronal excitation. The resulting perisomatic release of GABA generates IPSPs that briefly and rhythmically attenuate axonal plexus activity, thus phasing the excitatory inputs to interneurons and generating the gamma-frequency field oscillation. In a cyclical fashion, this interneuronal activity releases sufficient GABA to maintain ectopic rates in the plexus, thus establishing the persistent nature of the observed gamma oscillation. These data suggest, therefore, that neuronal networks tolerate random axonal activity only at levels below a certain threshold. When such random activity increases to a level above that threshold, for example when interneurons are active, networks impart temporal order on the resulting activity.
Acknowledgments
We thank the Medical Research Council (U.K.), The Volkswagen Stiftung, and the National Institute of Neurological Disorders and Stroke, National Institutes of Health, for supporting this work.
Abbreviations: EPSP, excitatory postsynaptic potential; GABA, γ-aminobutyric acid; GABAA and GABAB, GABA types A and B, respectively; IPSP, inhibitory postsynaptic potential.
References
- 1.Miltner, W. H., Braun, C., Arnold, M., Witte, H. & Taub, E. (1999) Nature 397, 434–436. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez, E., George, N., Lachaux, J. P., Martinerie, J., Renault, B. & Varela, F. J. (1999) Nature 397, 430–433. [DOI] [PubMed] [Google Scholar]
- 3.Csicsvari, J., Jamieson, B, Wise, K. D. & Buzsaki, G. (2003) Neuron 37, 311–322. [DOI] [PubMed] [Google Scholar]
- 4.Traub, R. D., Bibbig, A., Fisahn, A., LeBeau, F. E., Whittington, M. A. & Buhl, E. H. (2000) Eur. J. Neurosci. 12, 4093–4106. [DOI] [PubMed] [Google Scholar]
- 5.Hormuzdi, S. G., Pais, I., LeBeau, F. E., Towers, S. K., Rozov, A., Buhl, E. H., Whittington, M. A. & Monyer, H. (2002) Neuron 31, 487–495. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz, D., Schuchmann, S., Fisahn, A., Draguhn, A., Buhl, E. H., Petrasch-Parwez, E., Dermietzel, R., Heinemann, U. & Traub, R. D. (2001) Neuron 31, 831–840. [DOI] [PubMed] [Google Scholar]
- 7.Traub, R. D., Pais, I., Bibbig, A., LeBeau, F. E. N., Buhl, E. H., Hormuzdi, S. G., Monyer, H. & Whittington, M. A. (2003) Proc. Natl. Acad. Sci. USA 100, 1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draguhn, A., Traub, R. D., Schmitz, D. & Jefferys, J. G. (1998) Nature 394, 189–192. [DOI] [PubMed] [Google Scholar]
- 9.Traub, R. D., Schmitz, D., Jefferys, J. G. & Draguhn, A. (1999) Neuroscience 92, 407–426. [DOI] [PubMed] [Google Scholar]
- 10.Semyanov, A. & Kullmann, D. M. (2001) Nat. Neurosci. 4, 718–723. [DOI] [PubMed] [Google Scholar]
- 11.Morris, M. E., DiConstanzo, G. A., Fox, S. & Werman, R. (1983) Brain Res. 278, 117–126. [DOI] [PubMed] [Google Scholar]
- 12.Alford, S., Christenson, J. & Grillner, S. (1991) Eur. J. Neurosci. 3, 107–117. [DOI] [PubMed] [Google Scholar]
- 13.Sakatani, K., Black, J. A. & Kocsis, J. D. (1991) Proc. R. Soc. London Ser. B 247, 155–161. [DOI] [PubMed] [Google Scholar]
- 14.Stasheff, S. F., Mott, D. D. & Wilson, W. A. (1993) J. Neurophysiol. 70, 976–984. [DOI] [PubMed] [Google Scholar]
- 15.Avoli, M., Methot, M. & Kawasaki, H. (1998) Eur. J. Neurosci. 10, 2714–2722. [DOI] [PubMed] [Google Scholar]
- 16.Pinault, D. & Pumain, R. (1989) Neuroscience 31, 625–637. [DOI] [PubMed] [Google Scholar]
- 17.Whittington, M. A., Traub, R. D. & Jefferys, J. G. (1995) Nature 373, 612–615. [DOI] [PubMed] [Google Scholar]
- 18.Fisahn, A., Pike, F. G., Buhl, E. H. & Paulsen, O. (1998) Nature 394, 186–189. [DOI] [PubMed] [Google Scholar]
- 19.Deuchars, J. & Thomson, A. (1996) Neuroscience 74, 1009–1014. [DOI] [PubMed] [Google Scholar]
- 20.Miles, R. (1990) J. Physiol. (London) 428, 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulyas, A. I., Miles, R., Hajos, N. & Freund, T. F. (1993) Eur. J. Neurosci 5, 1729–1751. [DOI] [PubMed] [Google Scholar]
- 22.Traub, R. D. & Miles, R. (1995) J. Comput. Neurosci. 2, 291–298. [DOI] [PubMed] [Google Scholar]