Abstract
We assessed the relation between hemodynamic and electrical indices of brain function by performing simultaneous functional MRI (fMRI) and electroencephalography (EEG) in awake subjects at rest with eyes closed. Spontaneous power fluctuations of electrical rhythms were determined for multiple discrete frequency bands, and associated fMRI signal modulations were mapped on a voxel-by-voxel basis. There was little positive correlation of localized brain activity with alpha power (8–12 Hz), but strong and widespread negative correlation in lateral frontal and parietal cortices that are known to support attentional processes. Power in a 17–23 Hz range of beta activity was positively correlated with activity in retrosplenial, temporo-parietal, and dorsomedial prefrontal cortices. This set of areas has previously been characterized by high but coupled metabolism and blood flow at rest that decrease whenever subjects engage in explicit perception or action. The distributed patterns of fMRI activity that were correlated with power in different EEG bands overlapped strongly with those of functional connectivity, i.e., intrinsic covariations of regional activity at rest. This result indicates that, during resting wakefulness, and hence the absence of a task, these areas constitute separable and dynamic functional networks, and that activity in these networks is associated with distinct EEG signatures. Taken together with studies that have explicitly characterized the response properties of these distributed cortical systems, our findings may suggest that alpha oscillations signal a neural baseline with “inattention” whereas beta rhythms index spontaneous cognitive operations during conscious rest.
Keywords: electroencephalography, human brain, spontaneous activity, functional magnetic resonance imaging, blood oxygenation-level-dependent contrast
To “do and think nothing” is probably the hardest instruction to follow. By nature restless, we are driven toward activity when awake, and refraining from such activity is an efficient way of falling asleep. Despite its intrinsic instability, the “awake resting state” has been the most widely used experimental condition in functional neuroimaging studies. It usually serves to define a “baseline” of brain activity, and local task-related deviations from baseline values are interpreted as functional “activation” or “deactivation” in response to a precisely defined experimental condition (1). Because any such difference is meaningful only if both conditions compared are well defined, there is continuing interest in better understanding brain processes during the resting state.
From the earliest electrophysiological recordings, “spontaneous activity” has been observed in neuronal discharge patterns. Neurons fire not only in relation to a sensory or behavioral event but also variably and seemingly unpredictably including at “rest.” In the context of experiments that target stimulus-locked responses, such unpredictable activity has been considered “noise.” However, recent evidence suggests that spontaneous activity is coherently expressed in larger neuronal populations and functionally meaningful (2–4). But how are spontaneous fluctuations in activity organized macroscopically across the brain? Answering this question could enable us to better understand their functional significance. If, for instance, neural activity fluctuated coherently in specific brain circuits, it might be related to fluctuations of specific mental activities, the nature of which might then be inferred from existing studies reporting explicit activation of these circuits. This result would corroborate the concept of a “default mode” of brain function as proposed by others (5). The underlying idea is that when one is awake and at “rest,” brain activity switches to default processes, which are suspended when one is engaged in a task. However, demonstrating that during rest neural activity spontaneously fluctuates in specific distributed spatial patterns would also modify this concept. In contrast to a static baseline, rest would appear as intrinsically dynamic and different from other functional states by virtue of preferential association with a distinct subset of the totality of neural (and cognitive) processes.
Surprisingly, few studies have addressed this issue so far, despite the implications for interpreting functional neuroimaging studies. Here, we explored the neuroanatomical patterns of resting state fluctuations of human brain activity by simultaneously applying two neurophysiological recording techniques, functional MRI (fMRI) and electroencephalography (EEG). The contrast used in most fMRI studies, including the present one, is blood oxygenation-level-dependent (BOLD) (6). In recent visual stimulation studies combining microelectrode recordings and fMRI in anaesthetized monkeys, a linear correlation was found between the BOLD response and the stimulus-driven modulation of the local field potential, a measure of local synaptic activity (7). In relation to this electrical signal, the BOLD response is convolved with a spatio-temporal low-pass filter that reflects the properties of neurovascular coupling. In surface EEG, electrical signals arise from synchronization of postsynaptic potentials across large populations of cortical neurons (8). We used recent methodological developments that allow for continuous acquisition of EEG despite the artifacts generated by fMRI (9). Our starting hypothesis was that the dynamics of EEG activity at rest hold information about the functional state of subjects and can be used to dissociate different brain networks spontaneously engaged and disengaged in the absence of any explicit instruction or task.
Methods
Fifteen healthy volunteers (with written informed consent) were scanned during resting wakefulness on a 1.5-Tesla MRI system equipped with a gradient booster and by using a standard head coil (Siemens Vision, Erlangen, Germany). The instruction was to lie still with eyes closed and not fall asleep. Maintenance of wakefulness throughout the sessions was checked for (self-reported or sleep patterns on EEG), leading to the exclusion of 4 subjects. An additional subject was excluded because of poor data quality, and hence datasets of 10 subjects were analyzed (6 female, 4 male, aged 31 ± 3 yr). Each of two consecutive 20-min sessions per subject yielded 300 T2*-weighted echo-planar image volumes covering the entire cerebrum (voxel size 3.44 × 3.44 × 4 mm3, 19 slices with 1-mm gap in 2.8 s, volumes recorded every 4 s, echo time 50 ms).
EEG was recorded by using the BrainAmp MR EEG amplifier, brain vision recorder software (Brainproducts, Munich, Germany), and the BrainCap electrode cap (Falk Minow Services, Herrsching-Breitbrunn, Germany) at 29 positions (following the 10/20 system, sampled at 5 kHz, 0.016–250 Hz). This cap provides a reference position between Fz and Cz. Mastoid or ear electrodes would be susceptible to wire loops and effects from head restraining pads.
Off-line EEG signal correction was based on averaging and then subtracting imaging and pulse artifact (10, 11), as implemented in the brain vision analyzer (Brainproducts, Munich). In each session, the 300 EEG segments contaminated by imaging artifact were averaged. This first step is similar to recording evoked potentials, where time-locked averaging serves to identify a weak response embedded in the strong EEG signal. In this average, the non-locked EEG contribution to the signal zeroes out, and (different from evoked potentials) this average artifact signal is then subtracted from the artifact-laden EEG recorded originally, thus reconstituting the “true” biological EEG signal. This approach requires nonsaturating amplifiers and assumes constant artifact properties (Fig. 4, which is published as supporting information on the PNAS web site, www.pnas.org). To facilitate visual inspection of the corrected EEG, a 0.5-Hz high-pass and, due to residual high-frequency artifact from under-sampling, a 30-Hz low-pass Butterworth filter (48 dB) was used after the subtraction algorithm, precluding analysis of activity in the gamma range. Power spectrum analyses were performed by using a Fast Fourier Transform (1-s epochs, Hanning window). A more detailed description of these methods and their validation for our setting is reported elsewhere (12).
Image preprocessing [realignment, spatial normalization, and spatial smoothing with a 10-mm full width at half maximum (FWHM) Gaussian kernel], and statistical analysis were carried out by using the spm99 package (www.fil.ion.ucl.ac.uk/spm). Regressors for the model were derived from convolving the power time courses of the bands of interest (calculated from the raw amplitude mean of the two occipital EEG leads O1 and O2) with a canonical hemodynamic response function. They were then down-sampled to the frequency of image volume sampling and mean-scaled. For group analysis, a fixed-effects model was applied, and statistical inferences were corrected for multiple comparisons by using Gaussian random field theory. Responses were considered significant at P < 0.05, corrected, if confirmed in a random effects model at P < 0.001, uncorrected.
Results
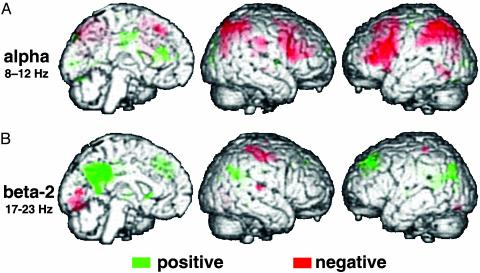
Like others (13), we first studied spontaneous fluctuations of “alpha” oscillations (8–12 Hz), the classical EEG hallmark of resting wakefulness with eyes closed (14). In other words, the power time course in this frequency band during a prolonged continuous resting state (convolved with the hemodynamic response function) served as a regressor for the analysis of the simultaneously acquired image data. Accordingly, positive correlation with alpha power should determine whether, and if so, which brain regions are more active when the brain expresses alpha oscillations than during alpha desynchronization. Positive correlation with alpha power was sparse and restricted to two foci in the cingulate gyrus and occipital cortex, but we found widespread negative correlation with alpha power in a bilateral fronto-parietal network (Fig. 1A and Table 1). This latter neuroanatomical pattern is well-known from functional neuroimaging experiments that overtly recruit attentional processes and related cognitive resources (15). We found no correlations between fMRI signal and power in the 4- to 7-Hz theta band.
Fig. 1.
Brain regions where BOLD fMRI signal is positively (green) or negatively correlated (red) with spontaneous power fluctuations in EEG frequency bands at rest (A,8–12 Hz; B,17–23 Hz). The results from a fixed effects group analysis (see Methods) are overlaid onto a rendering of a template brain and visualized at a threshold of P < 0.001, uncorrected. Note that activation in all clusters was also significant (P < 0.05) after correction for multiple comparisons, and see Table 1 for results at the level of a random effects analysis.
Table 1. Brain regions showing positive or negative correlation between BOLD fMRI signal and power in EEG frequency bands.
| EEG band | Brain region | Coordinates | Z-score | ||
|---|---|---|---|---|---|
| Alpha pos. | Occipital | -16 | -94 | 2 | 5.1 |
| Mid-cingulate | 8 | -14 | 44 | 3.9* | |
| Alpha neg. | Lateral prefrontal left | -44 | 34 | 16 | 5.8 |
| Lateral prefrontal right | 44 | 18 | 24 | 4.3 | |
| Parietal left | -50 | -52 | 50 | 4.2 | |
| Parietal right | 34 | -72 | 48 | 3.9 | |
| Beta-2 pos. | Posterior cingulate | 2 | -36 | 30 | 4.3 |
| Precuneus | -12 | -66 | 44 | 4.1* | |
| Temporo-parietal junction left | -36 | -62 | 36 | 4.5 | |
| Temporo-parietal junction right | 44 | -66 | 38 | 3.6* | |
| Dorsal medial prefrontal left | -16 | 38 | 28 | 3.8 | |
| Dorsal medial prefrontal right | 14 | 34 | 20 | 3.7* | |
| Beta-3 pos. | Anterior cingulate | 10 | 26 | 26 | 5.6 |
| Beta-3 neg. | Posterior cingulate | -4 | -46 | 18 | 4.1 |
| Temporo-parietal junction left | -34 | -80 | 26 | 3.7 | |
| Temporo-parietal junction right | 48 | -64 | 30 | 3.8 | |
The resuls are from a random effects analysis and list effects that were significant for single voxel peak height at P < 0.001 (with Z-scores reported) and for extent of the cluster at P < 0.01, both uncorrected (36). Asterisks indicate regions where the effects were too focal to fulfill the latter criterion.
Next, we analyzed activity fluctuations occurring in correlation with higher frequency oscillations in the “beta” band (13–30 Hz). We subdivided the beta range into three bands (16), and performed the same type of analysis as for alpha power, probing positive and negative correlations. No significant fMRI signal changes were associated with the beta-1 range (13–16 Hz), but we found positive correlation with beta-2 power (17–23 Hz) in several areas, namely the posterior cingulate and adjacent precuneus as well as the temporo-parietal junction and dorsomedial prefrontal cortex (Fig. 1B and Table 1). A trend for negative correlations with the beta-2 band was seen in primary cortices across the visual, auditory, and sensori-motor modalities, but the result did not pass the rigorous threshold criteria we set for statistical significance (see Methods). Finally, power fluctuations in the beta-3 band (24–30 Hz) were positively correlated with a region in the anterior cingulate gyrus and negatively correlated with retrosplenial and temporo-parietal as well as prefrontal areas. This observation suggested that similar areas were activating during high beta-2 and deactivating during beta-3 activity, a notion we submitted to formal testing by applying a conjunction analysis (Fig. 5, which is published as supporting information on the PNAS web site). The generalizability of the findings for both alpha and beta frequencies was confirmed in a random effects analysis (Table 1).
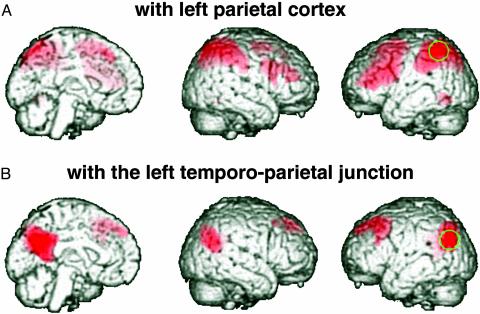
A remaining question was whether the correlation we observed between power fluctuations in certain EEG bands and activity fluctuations in several different brain areas was incidental, or whether it meant that EEG was signaling activity covariations in distributed but functionally connected systems. One could imagine finding correlations with EEG parameters across several areas even if they do not share the bulk of their variance over time. If so, our findings would probably be of low functional significance, and we would not be able to consider certain EEG bands to provide signatures of activity in distinct functional networks. We addressed the question of whether the distributed areas correlated with power in the same EEG band are indeed functionally coupled to each other by determining the intrinsic correlation patterns of spontaneous fMRI signal fluctuations. Others have used this approach as a measure of functional connectivity and have shown correlated signal changes during prolonged rest in the motor, auditory, and visual system (17–19). Here, we extracted the fMRI activity time courses from single voxels that significantly correlated with alpha (left parietal cortex) and beta-2 power (left temporo-parietal junction). By using these time series as regressors, we then mapped regions showing correlated signal changes in the group data set that we had previously analyzed in relation to the EEG findings (Fig. 2). This analysis demonstrated that virtually the same regions that were related to power changes in specific EEG bands were also intrinsically more strongly functionally connected to each other than to any other region. Moreover, our findings regarding the beta-2-associated network are in close agreement with a recent fMRI study on functional connectivity in the resting state (20).
Fig. 2.
Brain regions where BOLD fMRI signal at rest correlates with the reference regions indicated in green (stereotactic coordinates: A, –50, –52, 50; B, –36, –62, 36). The image data set is the same as in Fig. 1, and the functional connectivity results are visualized at a height threshold of T = 10.00.
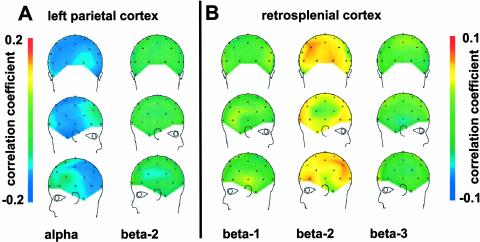
A further concern was that our results might have been influenced by the choice of the occipital EEG leads to provide the power time courses that we used as regressors for the fMRI analysis. We therefore took the reverse approach as in our first analysis and mapped the correlation of the band-specific power time courses at the different electrode positions with the fMRI activity time courses from different regions of interest (Fig. 3). We found that activity in different brain regions was linked to distinct EEG bands but not others, and that this correlation was not restricted to only a few electrode positions but observed for the majority. Thus, for instance, correlation of left parietal cortical activity with alpha power was negative across virtually all electrode positions (Fig. 3A), and no correlation was observed with the beta-2 band. Conversely, retrosplenial activity (from the posterior cingulate cortex) correlated positively with beta-2 power at most electrode positions but not detectably with the other beta bands (Fig. 3B). This finding underlines that the correlations observed between the frequency bands and distinct brain networks as monitored by the fMRI recording were not restricted to the electrodes chosen to provide the power time series. Due to high statistical interdependence of EEG signals across electrode positions we could not topographically assess the significance of these correlations. Conversely, collapsing the correlations across all electrode positions left little statistical power (one data point per subject and frequency band). Nonetheless, the difference between the correlations of left parietal BOLD signal with alpha and beta-2 power was significant, as was that between the correlation of retrosplenial activity with beta-2 and beta-3 power (P < 0.05 in one-sided paired t test after Fisher's Z-transformation). Of note, there were no negative correlations between the corresponding EEG power band time courses.
Fig. 3.
Mean correlation over all subjects of BOLD fMRI signal time courses with power changes in different EEG frequency bands for all scalp electrode positions. Brain activity data were derived from a volume of interest (5 mm sphere) around a voxel in the left parietal (A, stereotactic coordinates: 50, –52, 50) and retrosplenial cortex (B, stereotactic coordinates: 2, –36, 30). Note that, at the latter site, we observed no strong negative correlation of BOLD signal with the beta-3 band (compare location of peak effects in Table 1). Correlation with the individual power time courses was projected onto a standard template of the corresponding electrode positions.
Discussion
Together, the findings in this study generate a picture of intertwined yet dissociable dynamic brain processes occurring at conscious rest and suggest that, in the absence of defined experimental conditions, specific EEG bands provide signatures of activity in distinct networks of the human brain. Data from functional neuroimaging studies are usually interpreted in relation to stimulation characteristics or behavioral performance during specific experimental conditions. One of the problems when studying the resting state is the difficulty in positively and precisely defining its functional characteristics (1, 21). Most laboratories define rest operationally by the absence rather than the presence of factors that are known to influence brain activity. Accordingly, they minimize sensory input by blindfolding subjects or asking them to keep their eyes shut and by plugging their ears. By definition, instructions beyond “lie still and stay awake” are precluded because they would induce specific brain states instead of “rest.” The precise mental processes (and their timing) during rest hence remain essentially uncontrolled, and this is probably the main limitation to the utility of this condition as a “baseline” or “control.”
Despite this uncertainty regarding mental correlates, specific neuroanatomical activity patterns have been associated with resting wakefulness and thus define a functional baseline that is distinct from both sleep (22) and also any type of task involving explicit perception and action. This latter observation comes from metaanalyses of previous positron emission tomography measurements of blood flow at rest and during various experimental tasks (21, 23). “Reverse-subtraction” identified brain regions showing common, task-independent deactivations across many different experimental conditions when compared with rest. These conditions differed with respect to sensory input, motor output, and cognitive connotation, and, hence, consistently deactivated areas were considered more active at rest.
The question remains as to whether greater blood flow in certain brain regions during rest indicates an activation or simply a higher physiological baseline level that could putatively correspond to the continuous importance and prevalence of the mental operations these areas subserve (5, 24). An uncoupling of blood flow and oxygen consumption is often considered a label of activation, i.e., of deviations from a physiological steady-state baseline during which there is coupling (25). The oxygen extraction fraction (the ratio of oxygen consumption and blood flow and thus a measure of coupling) is widely homogenous at rest (5). This means that hemodynamic and metabolic measures are locally coupled over time and thus argues for an identity of the functional with the physiological baseline. Yet, the hemodynamic and metabolic activity values display (proportional) topographic heterogeneity. Remarkably, the highest absolute values of blood flow and metabolism at rest are found in retrosplenial cortex (5), an area that we observed to increase fMRI signal in correlation with beta-2 power. Very recently, an fMRI study on functional connectivity has obtained a very similar spatial pattern by analyzing fluctuations during the resting state (20). Because the fMRI signal in these studies arises from an uncoupling of blood flow from oxidative metabolism (26), those as well as our current findings for fMRI signal fluctuations show that greater activity at rest may at least in part reflect activation instead of only a higher physiological baseline level.
Moreover, the pattern of beta-2 associated activations we observed here during prolonged rest is highly congruent with the distribution of task-independent deactivations shown in the aforementioned “reverse-subtraction” analyses. This network includes posterior cingulate and precuneus as well as the temporo-parietal and dorsomedial prefrontal cortex (Fig. 1B). The brain structures composing this pattern have been implicitly (27, 28) or explicitly (24, 25, 29–31) linked to those cognitive processes that become active when one is put to rest, i.e., random episodic memory (23) and related imagery (30), conceptual processing (28), stimulus-independent thought (29), and self-reflection (24, 31). Together, these findings support the notion that the default mode of brain activity at rest has a specific functional connotation with cognitive and emotional processes revolving around the subject's internal state instead of current external events or circumstances (24). We identified power in the 17- to 23-Hz beta band as a signature of activity in the brain network that underlies this default mode of brain function.
In contrast to beta-2, high alpha power was not associated with activation except for two regions in the cingulate gyrus and some effect in occipital areas. Different from high-density EEG techniques (32), our recordings are probably mostly driven by global properties of alpha synchronization (12). If these global properties of alpha activity correlated with weak effects in broadly distributed neural populations, functional imaging might not be sensitive enough to detect significant activity changes on a voxel-by-voxel basis. Yet, the almost complete absence of focal activations during high alpha power also conforms to the classical understanding of alpha as the EEG rhythm “when the cortex has nothing to do” (33) and this pattern could hence be addressed as a “baseline mode.”
Alpha activity is blocked for instance by attentively processing external stimuli or during intentional mental operations with high cognitive load (8, 32). Accordingly, one could expect low alpha power to be associated with activations in cortical structures that orchestrate goal-directed cognition and behavior. Our findings are in good agreement with this notion: whenever alpha power decreased, the BOLD fMRI signal increased in frontal and parietal cortical areas that are involved in attention and related cognitive processes (15). We propose this to form a “set mode” of brain function that corresponds to abortive orienting reactions or loadings of working memory loops that occur (and subside) spontaneously during conscious rest. This finding is in line with one classical interpretation of the alpha “blockade” (most commonly elicited in the clinical EEG laboratory by eye opening) as an orienting reaction of the brain rather than a sensory process (34). That activations in the fronto-parietal cortices during rest have not been identified previously is probably due to the reverse-subtraction technique in which averaged brain activity during rest is compared with that during explicit cognitive tasks. Because these latter conditions probably recruit frontal and parietal cortex more strongly and continuously than this occurs during rest, reverse subtraction may fail to detect spontaneous activations in the attentional network.
The fact that the power time courses from different bands gave distinct results points to an at least partial independence of the associated networks. Moreover, the linkage of alpha activity to attention and of beta activity to cognition, respectively, is in line with long-standing EEG observations during mental processing (35). We do not believe that the link between EEG frequency spectrum and hemodynamic indices of synaptic activity as described here is direct. In other words, we do not consider our imaging data to delineate the neural sources of cortical alpha and beta oscillations, and it remains for future studies to clarify the relation between these two very different types of biological signal. Instead, our data suggest that cognitive function enters as an intervening variable between the two, and that attention or the “default mode” are associated with specific signatures both at the level of EEG and fMRI. Functional segregation of the associated neural systems was further confirmed by our analyses of functional connectivity (Fig. 2), and by using a reverse inference from localized brain activity to band-specific EEG patterns (Fig. 3).
As a practical consequence, the presence of alpha oscillations on surface EEG cannot serve to define a neural baseline for the entire brain but only for the structures that constitute the attentional system. During high alpha power, the beta-2-associated brain network (and perhaps other as yet unidentified systems) may or may not be active, thus precluding the definition of a global baseline from alpha power alone. Together, the dynamic approach to the resting state that we used here fosters an understanding of the nature and structure of spontaneous activity in the awake state. Our results illustrate that, during wakefulness, the brain never truly remains at rest. Instead of globally stabilizing at a homogenous baseline level, brain activity fluctuates within and between different modes that imply different segregated functional networks and have distinct EEG signatures.
Supplementary Material
Acknowledgments
We thank our volunteers for participation, the Institute of Neuroradiology for scanner access, and Anne-Lise Giraud and Richard Frackowiak for helpful comments. H.L. and the MRI-compatible EEG equipment are sponsored by the German Ministry for Education and Research (BMBF), K.K. is supported by a grant from the Frankfurt University Medical Faculty, and P.S., E.E., and A.K. are supported by the Volks-wagen Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: fMRI, functional MRI; EEG, electroencephalography; BOLD, blood oxygenation-level-dependent.
References
- 1.Gusnard, D. A. & Raichle, M. E. (2001) Nat. Rev. Neurosci. 2, 685–694. [DOI] [PubMed] [Google Scholar]
- 2.Arieli, A., Sterkin, A., Grinvald, A. & Aertsen, A. (1996) Science 273, 1868–1871. [DOI] [PubMed] [Google Scholar]
- 3.Tsodyks, M., Kenet, T., Grinvald, A. & Arieli, A. (1999) Science 286, 1943–1946. [DOI] [PubMed] [Google Scholar]
- 4.Leopold, D. A., Murayama, Y. & Logothetis, N. K. (2003) Cereb. Cortex 13, 422–433. [DOI] [PubMed] [Google Scholar]
- 5.Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A. & Shulman, G. L. (2001) Proc. Natl. Acad. Sci. USA 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa, S., Lee, T. M., Kay, A. R. & Tank, D. W. (1990) Proc. Natl. Acad. Sci. USA 87, 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. (2001) Nature 412, 150–157. [DOI] [PubMed] [Google Scholar]
- 8.Nunez, P. L., Wingeier, B. M. & Silberstein, R. B. (2001) Hum. Brain Mapp. 13, 125–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemieux, L., Salek-Haddadi, A., Josephs, O., Allen, P., Toms, N., Scott, C., Krakow, K., Turner, R. & Fish, D. R. (2001) Neuroimage 14, 780–787. [DOI] [PubMed] [Google Scholar]
- 10.Allen, P. J., Josephs, O. & Turner, R. (2000) Neuroimage 12, 230–239. [DOI] [PubMed] [Google Scholar]
- 11.Allen, P. J., Polizzi, G., Krakow, K., Fish, D. R. & Lemieux, L. (1998) Neuroimage 8, 229–239. [DOI] [PubMed] [Google Scholar]
- 12.Laufs, H., Beyerle, A., Eger, E., Salek-Haddadi, A., Preibisch, C., Kleinschmidt, A. & Krakow, K. (2003) NeuroImage 19, 1463–1476. [DOI] [PubMed] [Google Scholar]
- 13.Goldman, R. I., Stern, J. M., Engel, J., Jr. & Cohen, M. S. (2002) NeuroReport 13, 2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger, H. (1929) Archiv. Psychiatrie Nervenkrankheiten 87, 527–570. [Google Scholar]
- 15.Corbetta, M. & Shulman, G. L. (2002) Nat. Rev. Neurosci. 3, 201–215. [DOI] [PubMed] [Google Scholar]
- 16.Vogel, F. (1962) Dtsch. Z. Nervenheilkd. 184, 137–173.13997623 [Google Scholar]
- 17.Lowe, M. J., Mock, B. J. & Sorenson, J. A. (1998) NeuroImage 7, 119–132. [DOI] [PubMed] [Google Scholar]
- 18.Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. (1995) Magn. Reson. Med. 34, 537–541. [DOI] [PubMed] [Google Scholar]
- 19.Cordes, D., Haughton, V. M., Arfanakis, K., Carew, J. D., Turski, P. A., Moritz, C. H., Quigley, M. A. & Meyerand, M. E. (2001) AJNR Am. J. Neuroradiol. 22, 1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 20.Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. (2003) Proc. Natl. Acad. Sci. USA 100, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazoyer, B., Zago, L., Mellet, E., Bricogne, S., Etard, O., Houde, O., Crivello, F., Joliot, M., Petit, L. & Tzourio-Mazoyer, N. (2001) Brain Res. Bull. 54, 287–298. [DOI] [PubMed] [Google Scholar]
- 22.Maquet, P. (2000) J. Sleep Res. 9, 207–231. [DOI] [PubMed] [Google Scholar]
- 23.Shulman, G. L., Fiez, J. A., Corbetta, M., Buckner, R. L., Miezin, F. M., Raichle, M. E. & Petersen, S. E. (1997) J. Cogn. Neurosci. 9, 648–663. [DOI] [PubMed] [Google Scholar]
- 24.Gusnard, D. A., Akbudak, E., Shulman, G. L. & Raichle, M. E. (2001) Proc. Natl. Acad. Sci. USA 98, 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox, P. T., Raichle, M. E., Mintun, M. A. & Dence, C. (1988) Science 241, 462–464. [DOI] [PubMed] [Google Scholar]
- 26.Kwong, K. K., Belliveau, J. W., Chesler, D. A., Goldberg, I. E., Weisskoff, R. M., Poncelet, B. P., Kennedy, D. N., Hoppel, B. E., Cohen, M. S., Turner, R. & et al. (1992) Proc. Natl. Acad. Sci. USA 89, 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreasen, N., O'Leary, D., Cizadlo, T., Arndt, S., Rezai, K., Watkins, G., Ponto, L. & Hichwa, R. (1995) Am. J. Psychiatry 152, 1576–1585. [DOI] [PubMed] [Google Scholar]
- 28.Binder, J. R., Frost, J. A., Hammeke, T. A., Bellgowan, P. S., Rao, S. M. & Cox, R. W. (1999) J. Cogn. Neurosci. 11, 80–95. [DOI] [PubMed] [Google Scholar]
- 29.McGuire, P. K., Paulesu, E., Frackowiak, R. S. & Frith, C. D. (1996) NeuroReport 7, 2095–2099. [PubMed] [Google Scholar]
- 30.Fletcher, P. C., Frith, C. D., Baker, S. C., Shallice, T., Frackowiak, R. S. & Dolan, R. J. (1995) NeuroImage 2, 195–200. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, S. C., Baxter, L. C., Wilder, L. S., Pipe, J. G., Heiserman, J. E. & Prigatano, G. P. (2002) Brain 125, 1808–1814. [DOI] [PubMed] [Google Scholar]
- 32.Gevins, A., Smith, M. E., McEvoy, L. & Yu, D. (1997) Cereb. Cortex 7, 374–385. [DOI] [PubMed] [Google Scholar]
- 33.Adrian, E. D. & Matthews, B. H. C. (1934) Brain 57, 356. [Google Scholar]
- 34.Jung, R. (1953) in Handbuch der Inneren Medizin, eds. von Bergmann, G., Frey, W. & Schwiegk, H. (Springer, Berlin), Vol. 1, pp. 1216–1325. [Google Scholar]
- 35.Ray, W. J. & Cole, H. W. (1985) Science 228, 750–752. [DOI] [PubMed] [Google Scholar]
- 36.Poline, J. B., Worsley, K. J., Evans, A. C. & Friston, K. J. (1997) NeuroImage 5, 83–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.